Carbon Dioxide Sources and Sinks Activity
Jump to background information
Carbon dioxide (CO2) is an important gas in our atmosphere and is part of a natural biogeochemical cycle. Human activities, like burning fossil fuels, also emit CO2 into the atmosphere. The amount of CO2 in the atmosphere is increasing because of human activities, which have an impact on global climate. In this lesson, students will investigate some of the ways CO2 gets into and out of the atmosphere, and how this process might affect the overall balance in our world.
Learning Goals
- Students will be able to explain sources and sinks of carbon dioxide.
- Students will understand the relationship between the balance of CO2 sinks and sources and its effects on global climate.
Learning Objective
- Students will compare animal and fossil fuel sources of global carbon dioxide by using an indicator called bromothymol blue (BTB) to identify the presence or absence of carbon dioxide.
Materials
For each group of 3 students (parts 1 and 2):
- Safety goggles (one pair for each student)
- About 12 cm of masking tape (to make labels)
- Sharpie
- 6 test tubes
- One-hole test tube stopper with tubing attached (tubing long enough to fit into the bottom of a test tube)
- Test tube rack
- A small beaker of BTB solution
- A small beaker of vinegar
- A small sample of baking soda (about a half teaspoon)
- 1 2.5 cm x 2.5 cm square of foil
- 1 15 cm x 15 cm square of foil
- 3 cotton balls
- 1 straw
- 1 sprig of Elodea
- Student science notebooks (one per student)
- Carbon Dioxide Sources and Sinks Student Activity Sheet
For the class demonstration (part 3):
- 2 or more balloons
- One manilla folder
- Car (gasoline-burning)
- Duct tape
- Oven mitts
- Twist ties
Preparation
Launch and Investigate (Part 1 and 2)
- Prepare bromothymol blue (BTB) working solution according to product directions. Most test tubes hold 10-25 mL and each group will use a ⅓ test tube full for each of 4 investigations. Depending on the test tube size, each group will need 15-100 mL of solution. Divide solution into small beakers, one per group of 3 students.
- Prepare samples of vinegar for each group of students, ¼ test tube full for each group.
- Have available baking soda for student groups to collect. Each group will need approximately a penny-sized portion.
- Prepare one 2.5 cm square of foil for each group.
- Prepare one 15 cm square of foil for each group.
- Purchase Elodea (this can sometimes be found at a local pet store).
Develop (Part 3)
-
Advance preparation involves collecting automobile exhaust for the demonstration. This contains carbon monoxide (CO). Please be extremely careful while collecting this potentially harmful gas. You will need an assistant to help you.
Important note: Carbon monoxide is an odorless, moderately toxic, poisonous, and flammable gas. Teachers should provide students with balloons full of car exhaust. It is not recommended that students participate in filling the balloons with car exhaust. -
Blow up and allow the balloons to deflate. This will stretch the rubber and make them easier to fill with the relatively low-pressure exhaust.
-
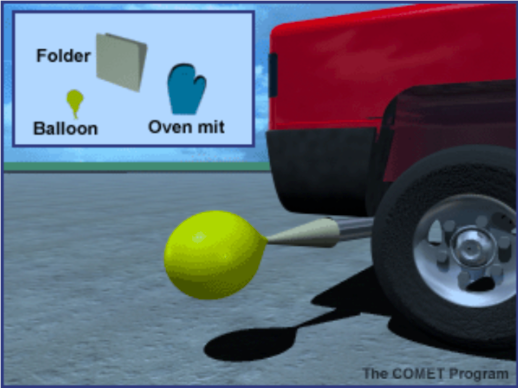
Prepare a cone to collect the car exhaust by rolling up a manila folder lengthwise. One end must be larger than the opening for the car’s tailpipe and the other end must be small enough for the balloon to fit over it.
-
Use plenty of tape to hold the cone in shape and to make the sides of the cone fairly airtight.
-
Have an adult assistant turn on the car. Make sure the brake is on and that the car is not in gear.
-
Put the balloon on the small end of the cone. Using the heat-resistant mitts, approach the exhaust pipe from the side. Place the large end of the cone over the tailpipe. Use the gloved hand to help form a seal between the cone and the exhaust pipe. DO NOT BREATHE THE EXHAUST. The balloon should fill quickly; if not, have your assistant step lightly on the accelerator.
-
When the balloon is filled, have an assistant use twist ties to tightly seal the balloon. Do this by twisting the neck several times and doubling it over once, then place the twist tie around the constricted area.
-
If you are doing this as a demonstration, have at least one extra balloon filled with exhaust. If students are doing this activity, have at least one balloon for each group of students. Ensure that this part is done in a well-ventilated room.
Directions
Launch
Ask students to brainstorm with a partner and write down everything they know about carbon dioxide. Then, ask each pair to contribute at least one idea to a whole class discussion. Write on the board student ideas, adding new ideas as each pair shares.
Project pictures of sources of CO2, including humans.
Investigate
Introduce the investigations by sharing with students they will discover the impact of carbon dioxide CO2 (and other greenhouse gases) in the atmosphere and its effect on climate.
Write the following questions on the board:
- Where does CO2 come from?
- How does CO2 get into the atmosphere? (source)
- What are ways that CO2 is taken out of the atmosphere? (sink)
Part 1: Detecting CO2
Combining baking soda (sodium bicarbonate, NaHCO3)and vinegar (acetic acid, HCH3COO) creates an acid-base reaction. One of the products formed is CO2. To test the BTB indicator, this reaction will be the source of CO2.
Ask students to create a T-chart in their science notebook as follows:
| CO2Source | CO2Sink |
Remind students that they will be making observations and taking notes in their science notebooks.
Assign students to groups of 3. Roles may be assigned: Equipment Manager, Facilitator/Data Collector, and Technician. Have the Equipment Manager collect materials. Students will prepare the materials and conduct the investigation as follows:
-
Put on your safety goggles.
-
With masking tape, label 5 test tubes A thru E (one test tube will be unlabeled).
-
Put test tubes A, B, and the unlabeled test tube in a test tube rack.
-
Fill test tubes A and B approximately 1/3 full with the BTB solution and place in the rack. Test tube A will be used as a control.
-
Fill the unlabeled test tube approximately 1/4 full of vinegar.
-
Using the foil, make a small “boat” for the baking soda. Fill 1/2 full of baking soda (hint: The ‘boat’ should be small enough to easily fit into the test tube and float on the vinegar).
-
Carefully slide the foil boat inside the unlabeled vinegar test tube (hint: tilt the test tube at an angle and carefully slide the boat in, being careful not to allow the baking soda and vinegar to make contact).
-
Plug the test tube with the stopper and tubing.
-
-
Place the free end of the tubing in test tube B, making sure the end of the tubing reaches the bottom of the test tube.
-
Place a cotton ball into the neck of test tube B.
-
-
Mix the vinegar and baking soda together by GENTLY swirling the unlabeled test tube from side to side Do NOT shake the test tube or turn it upside down.
Ask students to answer the following questions in their notebooks.
- What did you observe when the bubbles moved into the BTB solution in test tube B?
- Record your observations, adding both descriptions and sketches. (Students will see gas bubbles form and rapidly move out of the tubing into test tube B. They will see the color change in test tube B, indicating a presence of CO2.)
- How can you use BTB as an indicator for the presence of CO2?
Part 2: CO2Sources and Sinks: Animals and Plants
Have students continue to work with their groups. Students may switch roles. Each group will prepare the materials and conduct the investigation as follows:
-
Fill test tube C approximately 1/3 full of BTB.
-
Place a straw in the test tube.
-
Place a cotton ball in the test tube opening.
-
- Gently blow in the straw.
Ask students to answer the following questions in their notebooks.
-
What did you observe when you blew in test tube C? Record your observations, adding both descriptions and sketches. (Students will see the color change in test tube C, indicating the presence of CO2.)
-
How do the results of this investigation compare to Part 1?
-
Are animals a source of CO2? Describe how you know.
For the next section, make sure students set up the investigation before they leave for the day. Results take at least 24 hours. Students may again switch roles. Each group will prepare the materials and conduct the investigation as follows:
-
Fill test tube D approximately 1/3 full of BTB.
- Place a sprig of Elodea into the test tube(Hint: Use a pencil to push it all the way to the bottom of the tube).
-
Wrap the tube in foil so that no light can get in
- Place in test tube rack and leave for at least 24 hours.
At the start of class the next day:
-
Students should put on safety goggles, unwrap the foil, and record observations in their notebooks, adding both written descriptions and sketches.
-
Leave the unwrapped test tube D with Elodea in the light and observe what happens.
Ask students to answer the following questions in their notebooks.
-
What did you observe when you first unwrapped the foil from test tube D? Record your observations, adding both descriptions and sketches.
-
What did you observe when you put the unwrapped test tube in the light? Be descriptive.
-
Are plants a source of CO2? Explain your thinking based on your investigations.
Develop
Part 3: Sources of CO2: Fossil Fuels
Conduct the following as a whole-class demonstration. Ask students to use their notebooks to record observations, notes, and questions during the demonstration. Students should begin to see how the natural balance of CO2 can be disrupted by human activity.
-
Describe how you collected the contents of the balloon. Draw or project a picture. Ask students what they think came out of the exhaust of the car. Have them write a prediction in their notebooks about what will happen when you release the balloon’s contents into the BTB.
-
Fill a 500mL beaker approximately 1/2 full of BTB.
-
Take the exhaust-filled balloon and carefully untwist the tie while holding the neck of the balloon so that the gas does not escape. Twist and pinch the neck of the balloon to prevent air from escaping, but don’t tie it.
-
While still preventing the gas from escaping, insert a straw into the neck of the balloon up to the twisted portion. Seal the opening of the balloon tightly to one side by pinching it off with fingers. (Hint: Practice this a few times with a regular air-filled balloon.)
-
Insert the straw into the beaker.
-
Gently release air from the balloon by slowly untwisting the neck. Allow the gas to bubble out at a steady rate until the balloon is empty.
-
Provide each group with a sample of BTB from the beaker. Ask one student from each group to get their test tube E for the sample. Students will use this to compare to their other test tubes.
Ask students to answer the following questions in their notebooks. Then, have them create a poster, hang it on the wall, and share information with their classmates.
-
What was in the balloon?
-
What did you observe when your teacher released the balloon’s contents into the beaker? Use both a description and sketches.
-
Compare the colors in all of the test tubes, A-E. Are they different? If so, why? Explain your reasoning.
-
Have students draw a picture in their science notebooks showing how CO2 enters and gets out of the atmosphere. Instruct students to use the ideas from their investigations and the demonstration as they make the picture. Have groups discuss their pictures and create a poster that represents their ideas of CO2sources and sinks. Hang posters on the classroom wall. Have students visit other groups' posters and add any additional ideas to their notebooks.
Extension
Ask students to devise their own experiment to test other sources and sinks of carbon dioxide (e.g. carbonated beverages, lime-based chalk).
Assessment
Ask students to review all parts of this lesson and review their notes and observations in their science notebooks. Ask them to fill out the T-chart, indicating the different investigations with plants, animals, and fossil fuels. Encourage them to add any other resources they’ve studied or know about and add to their list of CO2 sources and sinks.
Then, ask them to answer the following questions.
-
Describe how the balance of CO2 changes due to human activity.
-
If you wished to reduce the amount of CO2in the atmosphere, which source would be most important to control? Why? How would you control it?
-
What would be some challenges with such controls? What would be the benefits?
Background
Carbon dioxide (CO2) has a characteristic that enables students to detect it in a classroom setting. When dissolved in water, carbon dioxide forms a weak acid, called carbonic acid. The chemical bromothymol blue (BTB) is a sensitive indicator of the presence of acid. When gas containing CO2 is bubbled through a BTB solution, carbonic acid forms and the indicator turns from dark blue to green, yellow, or very pale yellow depending on the CO2 concentration (lighter colors mean higher concentrations).
Carbon dioxide (CO2) provides the carbonation in soda pop and the “rise” in baked goods. But it is also a very significant greenhouse gas. CO2 is important in maintaining the Earth’s average temperature of about 15°C (59°F). The CO2 traps infrared energy emitted from the Earth’s surface and warms the atmosphere (gases surrounding the Earth). Without water vapor, CO2, and methane (the three most important naturally produced greenhouse gases), the earth’s surface would be about -18°C (0°F). At this temperature, it is doubtful that complex life as we know it would ever have evolved.
Where does CO2 come from? Plants and animals give it off when they extract energy from their food during cellular respiration. CO2 bubbles out of the earth in soda springs, explodes out of volcanoes, and is released when organic matter burns (such as during forest fires). Anything that releases CO2 into the atmosphere (living, dead, or non-living) is considered a source. Anything that absorbs and holds CO2 from the air or water is considered a sink (or holding reservoir). Plants and animals are sources, giving off CO2 while alive and respiring and when dead and decaying (bacteria that consume the dead bodies respire, too). Plants (both terrestrial plants and marine phytoplankton) are important carbon sinks, taking up vast quantities of CO2 through the process of photosynthesis. While plants also release CO2 through the process of respiration, on a global, annual basis, the amount of CO2 taken up by plants through photosynthesis and released through respiration approximately balances out.
Over geologic time, CO2 sources and sinks generally balance. In today’s atmosphere, however, CO2 levels are climbing in a dramatic and measurable fashion, providing evidence that their source of CO2 is producing CO2 at a faster rate than sinks can remove it. Human activities, such as the burning of fossil fuels (coal, oil, gas) have increased the concentration of CO2 in the atmosphere. This change in concentration of greenhouse gases contributes to global climate change because these gases trap heat. Consequences include warming of global climate and regional changes in precipitation, rising sea levels, melting glaciers and sea ice, changes in plant communities, species extinctions, and more.