Detecting Ultraviolet Light Using Tonic Water
In this activity, students use a model to test actions for staying safe from the Sun's ultraviolet radiation.
Learning Objectives
- Students will understand that UV radiation is a part of the Sun's electromagnetic spectrum, which has shorter wavelengths than visible light.
- Students will be able to describe ways to keep skin safe from UV radiation and which methods are most effective.
- Students will design and execute an experiment to test a question about effective methods of blocking UV radiation using a model.
Materials
- For each student:
- Reading: EPA Sun Safety Action Steps
- Science notebook or paper to record observations
- Pencil
- For each group of 3-4 students:
- Poster paper, glue, and markers
- Or, a computer with presentation software like PowerPoint
- For the class:
- A blacklight
- 2 clear plastic cups*
- One can or bottle of tonic water*
- A piece of dark cloth to darken the lamp and cups
- Sunscreen (the spray variety is recommended
- Clear plastic bags or overhead transparency sheets
- Types of fabric/clothing typically worn to cover up from the Sun
- Shrub or tree branches with leaves
- Other items that are used for shade such as umbrellas
- Blue colored pencils and/or cameras
- Piece of glass
*Note: Frisbees that change color in UV light can be used instead of cups of tonic water in this activity.
Preparation
- Provide the EPA Sun Safety Action Steps reading for students, either as a handout or on computers/tablets.
- Set up the blacklight in the classroom where all students can see it. Fill two cups with tonic water and place them in front of the black light. (You do not need to turn on the blacklight until it is time to demonstrate.)
Directions
- Introduce the activity, telling students that they will explore ultraviolet wavelengths of sunlight, which are dangerous and cause skin damage.
- Survey student knowledge of ultraviolet (or UV) light and student experience with Sun damage such as sunburns and skin cancer.
- Explain that different types of light are part of the electromagnetic spectrum. UV light has a wavelength slightly shorter than visible light. Unlike visible light, we can’t see UV, but some materials react in its presence. An ingredient in tonic water, called quinine, absorbs UV and releases it, causing it to glow blue (or fluoresce). Demonstrate how tonic water fluoresces when the black light is turned on. Tell students that the blacklight emits UV light, not visible light, so it doesn’t look bright to us.
- Explain to students that ultraviolet light is dangerous to humans. Show students a photo of someone with a sunburn or skin cancer. Explain that people with darker skin tone are not as likely to get sunburns as people with lighter skin tone, but everyone is vulnerable to skin damage from UV radiation, no matter what their skin color.

(A) set-up with blacklight and two cups of tonic water, (B) darkened classroom with blacklight on and tonic glowing blue as quinine absorbs and then releases UV light
Credit: Lisa Gardiner/UCAR
- Have students read the EPA Sun Safety Action Steps and list the steps that are recommended for staying away from UV light from the Sun.
- Ask students whether they think some actions described in the reading are more effective than others. Have each group of 3-4 students brainstorm ten questions that they have about the effectiveness of different actions described in the reading. (For example, students may wonder how effective it is to stay in the shade of a tree. They may wonder what types of clothing are best for covering up or whether the amount of sunscreen makes a difference.)
- Tell students that they are going to design an investigation to test one of these questions. They will use the blacklight to model ultraviolet light from the Sun and test how much the tonic water fluoresces when different actions are taken to block the UV light.
- Demonstrate how actions to block ultraviolet light can be explored using tonic water. For example, ask students if they think UV light can be transmitted through the windows of the classroom like the visible light is transmitted. Place a piece of glass between the black light and one of the cups of tonic water. The fluorescence should stop for the blocked cup (glass typically doesn’t transmit UV) but not for the other cup. Tell students that the items described in the reading (clothing, sunscreen, tree branches) can be put between the light and tonic water like “screens” to assess whether the UV light is transmitted through them. Point out that the amount of fluorescence is relative, so students will need to compare with the unblocked cup.
- Have students work in their groups to decide which of their ten questions they would like to test and plan the investigation. To communicate their plan, have students write:
- the question they will explore
- the supplies they will need for their “screens”
- the method they will use to test their question
- Review each group’s project plan noting if the method will address the question and whether supplies are practical. (Students might need advice about supplies and methods.)
- Have student groups make the “screens” that they will place between the light and tonic water. (Note: See About Making “Screens” in the Background section for information and ideas.)
- Have students test their screens between the light and the tonic, recording the difference in fluorescence with photos or by documenting the blue florescence in their notebooks with colored pencils. Have students try their screens multiple times to see if the results are consistent. (This data is qualitative, thus students will need to describe the amount of blue fluorescence from the tonic water relative to other amounts.)
- Have students create a graphic to describe their data and make a poster (or a presentation slide) with their group’s question, their graphic results, and their conclusion.
- Lead a gallery walk of the posters in which students review the results of all groups. (If students have made slides instead of posters, have each group present in front of the class.)
- Have students discuss what they learned about the best ways to stay safe from UV light. If time allows, have students consider whether they have more questions about staying safe from UV light after the experiment.
Background
The energy from the Sun includes not only visible light but also longer (infrared) and shorter (ultraviolet) wavelengths. The wavelengths of visible light increase from violet to red across the spectrum. Shorter than violet are wavelengths referred to as ultraviolet radiation (UV). "Ultra" means beyond, so ultraviolet means beyond (actually, shorter than) violet.
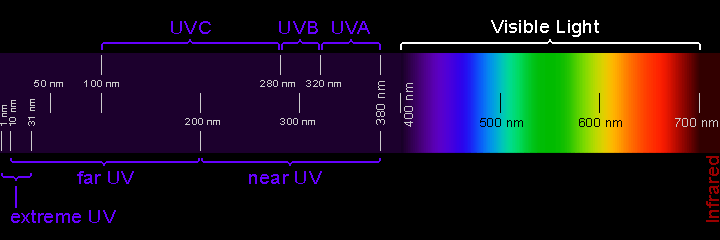
Wavelengths of ultraviolet, visible, and infrared light, in nanometers (nm)
Credit: Randy Russell/UCAR
The amount of UV radiation reaching the Earth's surface at a particular point depends on the distance it travels through the atmosphere. During morning hours, UV radiation must travel through more of the Earth's atmosphere because the Sun is lower on the horizon. At solar noon, the rays travel a shorter distance through the atmosphere because the Sun is more directly overhead
This activity used tonic water to demonstrate UV light presence. When a photon of UV energy is absorbed by the quinine, it is reemitted in tonic water as a photon of visible light. This process is called fluorescence. The extent of fluorescence that occurs is related to the amount of UV light.
About Making “Screens”
In this activity, students make "screens" to put between the blacklight and tonic water, testing what materials prevent UV transmission. For some materials (like clothing) making a screen will be straightforward. Other materials (like leaves of a tree or sunscreen, described below) will require a little engineering.
- If students wish to test the effectiveness of sunscreen, they can spread it on clear sheets of plastic (such as overhead transparencies) or plastic bags. It’s easy to get consistent coverage with spray sunscreen. Be sure to check and make sure the plastic, itself, does not block UV by putting it, without sunscreen, between the blacklight and tonic water and noting that it does not change the amount of fluorescence.
- For tree shade, consider having students observe how dense the leaves are in trees outside, taking photos of the canopy for reference. They can make a screen out of smaller leaves to use in the model, spacing them as they observed in real trees.