Waves of Energy More or Less
Students create and observe wavelengths at both high and low energy levels using safety glasses, rope, and a power drill.
Learning Objectives
- Students will be able to explain that energy travels from the Sun to the Earth by means of electromagnetic waves.
- Students will understand that shorter wavelengths have higher frequency and energy.
- Students will understand that longer wavelengths have lower frequency and energy.
- Students will be able to explain why shorter wavelengths carry more energy than longer wavelengths.
- Students will be able to demonstrate how a wavelength is measured.
Materials
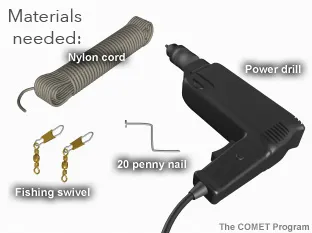
- Power electric drill and chuck key
- One 20 penny nail, bent in vise with pliers
- Nylon cord; cut one piece to 15 feet and another to 1 foot
- Two #2 fishing swivels (found in fishing section of sporting goods)
- Toy Slinkies or thin rope (optional)
Directions
- Before presenting this demonstration, provide some background information on the electromagnetic spectrum.
- Prior to the demonstration, you will need to bend a 20-penny nail as shown. You will have to use a vise and pliers to accomplish this.
- Attach a swivel to each end of the nylon cord.
- Tie the 1-foot piece of cord to one of the swivel holders. This is the piece of cord that a student will hold during the demonstration.
- Slide the bent nail through the eye of the other swivel.
- The nail end should be put into the drill bit fitting and tightened securely with the chuck key.
- To ensure the safety of your students, it is imperative that the cord not break during the demonstration. Be sure to test it before you present it to your students. Also, ensure that students helping with the demonstration are wearing safety glasses.
- Ask a student to hold the end of the cord that is not at the drill at a distance so that the cord has some tension.
- Plug in the drill, turn it on, and the demonstration begins. The less tension you apply, the more waves will appear. You can also vary the speed and reverse the direction of the drill to get different wave effects. Experiment and have fun! Note: If you do not want the complication of using a drill, you can simply have students use a length of rope or a Slinky to demonstrate energy and wavelengths.
Ask students the following questions
- What happens to the length of the wave when the drill speeds up, i.e., when more energy is added? (The wavelength shortens.)
- What occurs to the length of the wave when the drill is slowed? (The wavelength lengthens.)
- What does the length of the wavelength convey? (Short wavelengths have more energy, while long wavelengths have less energy.)
- UV radiation has a relatively short wavelength, shorter than visible light. What is the energy of UV radiation compared to visible light? (UV has higher energy.)
- What about infrared radiation? How does it compare to visible light? (It has a longer wavelength and has less energy.)
- Looking at an illustration of the electromagnetic spectrum, what type of radiation has the shortest wavelength, and what does that signify? (Gamma rays have short wavelengths, and thus have very high energy, which can be damaging to life on Earth.)
- How is wavelength measured? (Wavelength is measured in unit of distance between two wave crests.
- How is wave frequency measured? (Frequency is number of complete waves passing per unit of time. It is measured in Hertz (Hz), the number per second.)
Note: Students of all levels should be able to answer the first three questions and secondary level students should be able to answer the other questions too.
Background
Sunlight is a part of the electromagnetic spectrum. There are also parts of the electromagnetic spectrum that are less familiar to many of us. Some have more energy and some have less. From highest to lowest energy levels, these sources from the Sun include gamma rays, x-rays, ultraviolet rays, visible light, infrared rays, and radio waves (see graphic below). Microwaves are a subset of radio waves.
Energy is transported in waves. Light is made of discrete packets of energy called photons that travel at the speed of light until they come into contact with other matter. Photons have no mass, but they do have momentum. Likewise, energy from the electromagnetic spectrum has both particle-like and wave-like properties. Electromagnetic waves are formed by the vibrations of electric and magnetic fields. These fields are perpendicular to one another in the direction the wave is traveling.
The number of crests that pass a given point within one second is described as the frequency of the wave and is measured in Hertz (Hz). Electromagnetic waves have crests and troughs with the distance between crests called the wavelength. Wavelength is measured as a unit of distance such as mm, cm, nanometer, micrometer, etc.
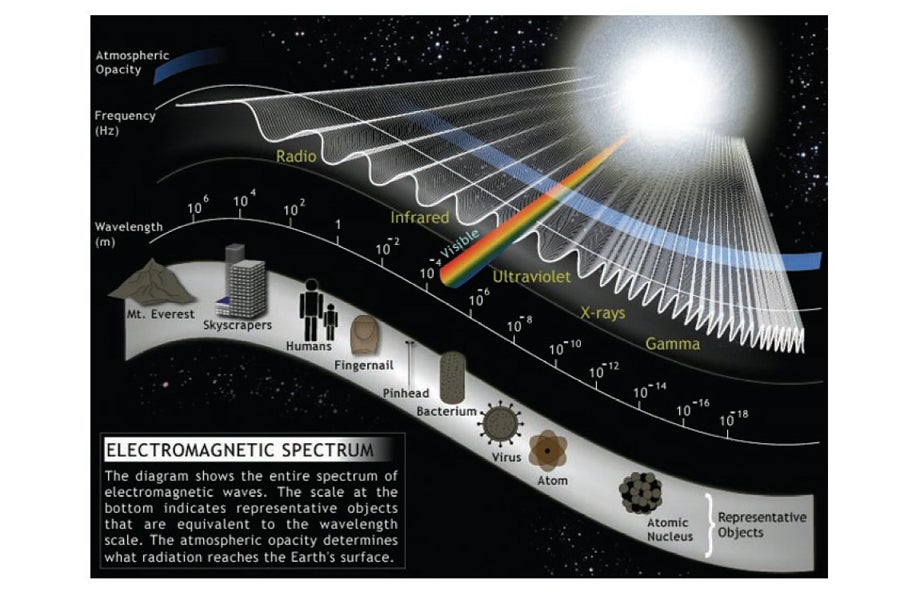
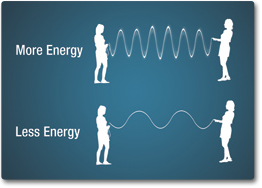
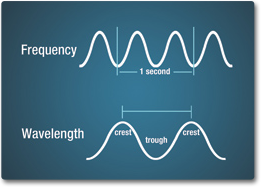
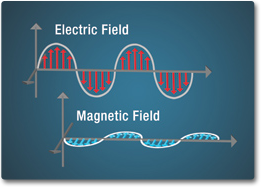
Images Source: NASA
Related Resources
- See COMET's MetEd resources for a more indepth explanation of the electromagnetic spectrum. (You can sign-in by creating a free account.)