Jim Smith - Atmospheric Chemist
Measuring minuscule particles in the air

Jim Smith
Carlye Calvin, UCAR
"It's fun to make something happen that at first glance seems impossible," says Jim Smith.
An atmospheric chemist at NCAR, Jim studies ultrafine aerosols. He and other aerosol scientists used to think it would be impossible to ever measure the chemical composition of these tiny particles that abound in the air. At less than 100 nanometers, the particles are about one thousandth the diameter of a strand of human hair, making them extremely difficult to measure.
For the past five years, Jim and his colleagues have been consumed by the development of an instrument known as a thermal desorption chemical ionization mass spectrometer, or TDCIMS. The innovative spectrometer can measure the chemical composition of particles as small as 4 nanometers. It works by giving the particles an electric charge and collecting them on a metal filament. Subjected to heat and chemical ionization, the particles turn to gas and can be analyzed with a mass spectrometer.
Jim is most interested in newly-formed ultrafine aerosols that span a mere 4 to 20 nanometers. "These are the smallest particles that we have yet to characterize in the atmosphere," he says. "They're not too far from being molecules." He describes the ability to measure their organic compounds as the "Holy Grail" of his specialization. "It's one of the toughest measurements to make, but we're optimistic."
Ultrafine aerosols come from automobile and power plant emissions as well as from natural sources like trees and vegetation. They are important because despite their minuscule size—or more precisely, because of their size—they affect both global climate and human health.
When you take a deep breath of air, you probably inhale more than a half million of these particles. Because they are so small, the particles can travel through cell walls and damage lung tissue and even move around in the body. "A lot of our work is applicable to human health," Jim notes.
In the atmosphere, water can cling to the tiny particles, forming cloud droplets. "The particles affect cloud processes by increasing the concentration of cloud condensation nuclei," Jim explains. Cloud condensation nuclei are small particles on which cloud droplets coalesce. Clouds in turn have a major impact on climate.
Jim and his colleagues have used TDCIMS to analyze particle composition in real time at NCAR's Marshall Field Site. They took the spectrometer to Atlanta on a field project in 2002 to analyze the chemical composition of newly-formed aerosols that measured about 7 nanometers. Next spring, Jim will take TDCIMS to Mexico City during the MIRAGE campaign to study the composition of urban aerosols, and then spend four months in Finland using the spectrometer to look at the formation of aerosols in forests.
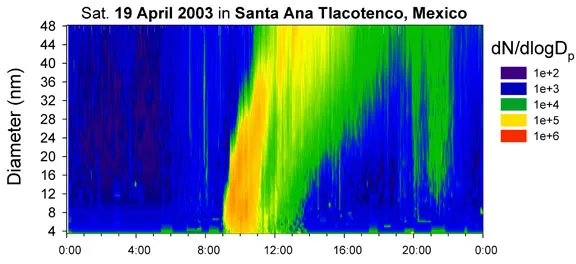
Jim uses the TDCIMS to study particle formation in the atmosphere, such as the event shown here from Santa Ana, Mexico, a small town that lies near the southeastern border of Mexico City and on the western rim of a mountain pass that channels the southern outflow of air from the city. The image, captured by a scanning mobility particle sizer, shows a 24-hour plot of particle diameter (vertical axis) versus particle concentration (with warmer colors represent higher concentrations). The plot is dominated by a new particle-formation event that began at 9:00 a.m. The new particles grew over time through the uptake of gases such as sulfuric acid, reaching diameters of 40 nanometers and above by 2:00 p.m.
Jim Smith, NCAR
Jim grew up in San Jose, California. His father was a ranger in Yosemite National Park, so the family spent summers rambling in the backcountry. "I was raised with a focus on the natural world," he says. "I knew that whatever field I went into would have something to do with nature."
Jim was also aware that science was his strong subject in school. He especially enjoyed physics in high school and went on to major in the subject at Harvey Mudd College in Claremont, California. After graduation, as most of his classmates headed off to graduate school, he went to Switzerland and worked as a machinist for a year. "I came home after that year and decided I still wasn't ready for grad school," he recalls.
Instead, Jim landed a job in the San Francisco Bay area as the first full-time employee of a new company called Aerometrics that was developing instruments to measure the size of particles based on how they scatter light. He ended up staying at the company for seven years before deciding that if he wanted to devote himself to studying atmospheric particles, he needed an advanced degree.
Since he had already looked at the optical properties of aerosols while at Aerometrics, Jim switched gears and focused on their chemical properties for his Ph.D. in environmental engineering science at California Institute of Technology. He says that although most people begin Ph.D. programs within a few years after college, he's glad he worked for seven years in between. "I came into grad school with a practical and well-organized approach to my work, and it set the tone for the rest of my years there," he reflects. People often assumed that as an older student he missed his steady job and salary, but after working for so many years he truly appreciated the time and flexibility of student life.
After his Ph.D. and a postdoctoral appointment in Sweden, Jim came to NCAR in 2000 to work as a postdoctoral fellow before joining the Atmospheric Chemistry Division. He says that he can see himself in the distant future doing exactly what he does now. "To stay motivated I take advantage of all NCAR's collaborative programs and work with others in the community," he says. "The people are the important thing."
by Nicole Gordon
September 2005