It's Just a Phase: Modeling the Phases of Water
In this activity, students will construct models of the arrangement of water molecules in the three physical states. Students will understand that matter can be found in three forms or phases (solid, liquid, and gas). Using physical models, students will be able to explain the molecular behavior of ice, water, and water vapor.
Learning Objectives
- Students will understand that matter can be found in three forms or phases (solid, liquid, and gas).
- Using physical models, students will be able to explain the molecular behavior of ice, water, and water vapor in relation to temperature.
Materials
- Petri dishes
- Tape
- BBs (or roughly spherical beads)
- Overhead projector
Preparation
- Hand out three Petri dishes to each student group (3 students per group). Plastic Petri dishes work best.
- Provide BBs or roughly spherical beads to each student group.
- Prepare overhead projector for class to share.
Directions
- Introduce the activity.
- Help students think about what they already know about the states of matter and the phases of water. For example, ask students, "When ice melts, what happens? What is the new phase of water?" and "When liquid water is heated up and the water is evaporated into the air, what is this new phase of water?"
- Explain that, in this activity, they will explore the phases of water (solid, liquid, and gas) by developing a simple model.
- Discuss the phases of water using the provided information and graphics in the Background Section. Also, discuss how the phases of matter (water) depend on the temperature (added or removed thermal energy).
- Make sure to give the students some understanding that water molecules themselves don't change when the state of the matter changes, but that the interaction between molecules changes.
- Explain to the students that a water molecule is made up of two hydrogen atoms and one oxygen atom. To illustrate what a molecule looks like, ask each student to visualize his or her head as being the oxygen atom with their two fists representing the hydrogen atoms.
- Teacher hands out each student team three Petri dishes, a supply of BBs or beads (BBs used for this example), and some tape.
- Challenge the students to imagine that each dish is a VERY enlarged part of a phase of water: one dish is ice, one is liquid, and one is vapor. Explain that the BBs represent the water molecules themselves.
- Tell the students to model what the molecules would look like in each phase (ice, liquid, water vapor). Also, have the students place the Petri dishes in order from the coldest to warmest temperature (left to right).
- If students need a hint, ask "In which form are the molecules most tightly held together? and "What would that look like in the Petri dish?"
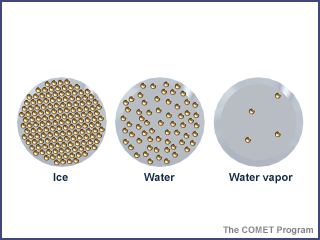
- Typically, students will arrive at similar models within a short time that look something like this
- Notes:
- Allow the students to produce the models as they see fit, but then challenge them to explain, from their model, how ice floats in water (presumably the students will be familiar with the concept of density, and with the fact that less dense things float on more dense things). They should recognize that the packed BBs in the ice model are too dense to float in water!
- While generally accurate for liquid water and water vapor, the model is wrong for ice, though it reflects a very common misconception about the structure of ice. Most students would explain that solid things are always denser than liquid and gaseous things, forgetting that water is a powerful and important exception to this rule.
- Place Petri dishes on an overhead projector for class share and jiggle it to show how the molecules move, or in the case of the ice model, don't move.
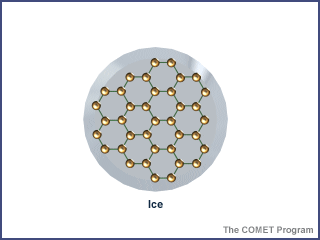
- Optional extension: As a separate class demo, the teacher prepares Petri dish ahead of class to illustrate the density of ice, with drops of superglue molding the BBs onto the dish such that they are spread out more than in the water. Next, you should draw lines between the BBs on the dish, indicating that the BBs are bonded together as illustrated below.
- Optional extension (weather permitting): Take the class of students outside and divide them into groups of six to eight. Have the students demonstrate how their group would look if they were water molecules in the three different phases.
- As a class, discuss what the water molecules looked like in each phase of the activity. Students (of all ages) often think that "liquid water molecules" are different from "ice molecules" or "vapor molecules." Emphasize that the molecules DO NOT change, only their arrangement, and that the same holds true for all other matter as well.
Assessment
- Observe the groups as they work in the lab or outside to identify students who are having conceptual troubles.
- At the end of the lesson, give students yet another model idea to diagram and explain individually. For example:
- Imagine that the closet is an ice cube and that the water molecules are red balloons. If you open the closet door, what will you see?
- Now, using red balloons as molecules again, imagine that the closet is full of liquid water.
- What will be different when you open the door?
- How will it differ if there's water vapor in the closet instead?
- Have students draw diagrams and/or explain on a separate sheet of paper and turn it in.
Modifications for Alternative Learners
- Make sure that students can use labeled diagrams without abundant text for any written report or assessment
Background
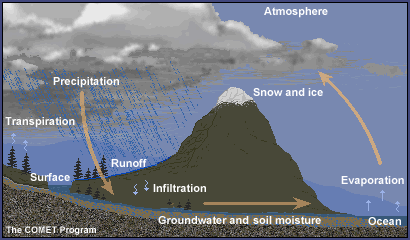
Water is an essential part of the earth system. Water is special not only because it covers over 70% of the earth's surface, but also because it is the only known substance that can exist in gaseous, liquid, and solid phases within the relatively narrow range of temperatures and pressures found on Earth.
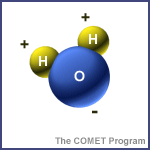
Water's special qualities come from the unique shape of the water molecule. Each molecule contains two atoms of hydrogen and one atom of oxygen, arranged such that one side of the molecule (nearest the hydrogens) is positively charged while the other side (nearest the oxygen) is negatively charged. If two water molecules come together, the positive side of one is attracted to the negative side of the other, making the molecules cling together. This simple fact accounts for the high heat capacity, surface tension, cohesion, adhesion, and other characteristics that make water so important to the earth's biosphere.
In general, when considering the states of matter, solids are denser than liquids and liquids are denser than gases. Water is a bit of a contrarian in this regard. In addition, the phases of matter (water) depend on the temperature or thermal energy. Adding or removing thermal energy increases or decreases the kinetic energy of the particles (in this case, water particles). The added heat or thermal energy leads to the molecular bonds breaking which leads to a change of state of solid to a liquid, then eventually gas. Solids melt when they absorb enough thermal energy. In contrast, when a liquid is cooled, thermal energy is released and reverts back to solid form. On the molecular level, when in solid form (liquid water), particles have less energy/movement but the particles have more energy and are more spread out in gas form (water vapor).
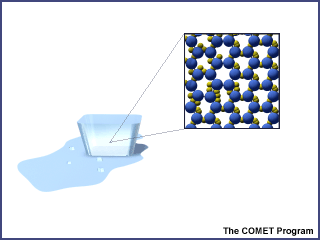 When water is in its solid-state (ice), the water molecules are packed close together preventing it from changing shape. Ice has a very regular pattern with the molecules rigidly apart from one another connected by the hydrogen bonds that form a crystalline lattice. These crystals have a number of open regions and pockets making ice less dense than liquid water. This is why ice floats on water. Ice forms when the temperature is below freezing (0° Celsius or 32° Fahrenheit).
When water is in its solid-state (ice), the water molecules are packed close together preventing it from changing shape. Ice has a very regular pattern with the molecules rigidly apart from one another connected by the hydrogen bonds that form a crystalline lattice. These crystals have a number of open regions and pockets making ice less dense than liquid water. This is why ice floats on water. Ice forms when the temperature is below freezing (0° Celsius or 32° Fahrenheit).
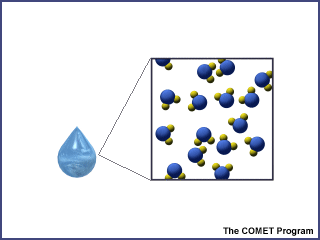
When ice is warmed above freezing, it melts and becomes liquid water. As a liquid, the attractive forces between molecules weaken and individual molecules can begin to move around each other. Because the molecules can slip and slide around one another, water takes the shape of any container it is in.
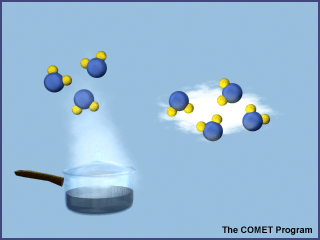 The third state of water is the gaseous state (water vapor). In this state, water molecules move very rapidly and are not bound together. Although we cannot see water in its gaseous state, we can feel it in the air on a hot, humid day. Commonly, water boils at a temperature of 100° C (212° F), forming water vapor. Many people believe that the visible plume of steam from a boiling kettle is water vapor. However, the steam that you see consists of very small water droplets suspended in the air, while water vapor is the invisible gas that results when water evaporates. We can "see" water vapor through the electromagnetic eyes of infrared-sensing instruments.
The third state of water is the gaseous state (water vapor). In this state, water molecules move very rapidly and are not bound together. Although we cannot see water in its gaseous state, we can feel it in the air on a hot, humid day. Commonly, water boils at a temperature of 100° C (212° F), forming water vapor. Many people believe that the visible plume of steam from a boiling kettle is water vapor. However, the steam that you see consists of very small water droplets suspended in the air, while water vapor is the invisible gas that results when water evaporates. We can "see" water vapor through the electromagnetic eyes of infrared-sensing instruments.
Water cycles endlessly throughout the atmosphere, oceans, land, and life of planet earth, taking each physical state at one time or another.