Radiation and Albedo Experiment
Students will investigate how different surfaces of the Earth reflect and absorb heat and apply this knowledge to real-world situations.
Learning Objectives
- Students will understand that the physical characteristics of the Earth's surface affect the way that surface absorbs and releases heat from the Sun.
Materials
For a class demonstration or for each team of students:
- Three pie pans or dishes
- Dark potting soil
- Light-colored sand or perlite
- Water
- Three thermometers
- Reflector lamp within can descent light bulb
- Watch with a second hand
For each student:
- Graph paper
- Colored pencils
Preparation
- Print the Heating and Cooling Data Tables found in the "Student" tab on this activity page.
- This activity can be done as a class demonstration or in groups of 3-4 students. If you are doing it as a demonstration, you'll need one set of materials and graph paper and data tables for each student. If the activity will be done in small groups, each group will need supplies.
- Check the light bulbs to ensure they generate heat. Use incandescent light bulbs instead of LED or CFL bulbs.
Directions
- Introduce the activity.
- Help students think about what they already know about how the color and type of material affects how hot it gets in the sunshine. For example, ask students, "When it is a hot day, what color shirt would you wear to keep cool and why? and "During the summer, what would it feel like to walk on asphalt or gravel with no shoes?"
- Explain that, in this activity, they will explore how different types of surfaces found at the Earth's surface (such as sand, soil, and water) heat up when the Sun's energy reaches them, and how they cool down when out of the sunshine.
- Note that this experiment uses materials to model sunshine and Earth materials. Show students the materials and explain how each relates to the Earth system. (The lamp represents the Sun in this model. The sand represents beaches, sand dunes, and rocks. The potting soil represents large areas of soil outdoors. And the water represents lakes, rivers, and the ocean.)
- Fill the pie pans to the same level, one with dark soil, one with light sand, and one with water. (If small groups are doing this activity on their own, distribute supplies to students.)
- Place the pie pans on a table or desk and position the lamp about 12 inches above them. (Do not turn on the lamp yet.)
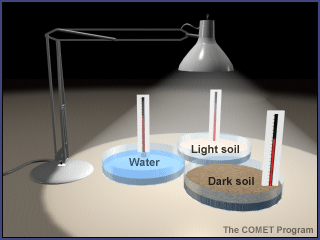
- Place a thermometer into each pie pan, with the bulb just under the surface of the substance (soil, sand, or water) in the pan.
- Provide students with data tables and explain how the tables relate to the experimental design.
- Have students record the temperature right before they turn on the lamp (Time=0), entering the number into their data table.
- Turn on the lamp and instruct students to record the temperature every minute in the three pie pans for ten minutes.
- After ten minutes, turn the lamp off and instruct students to continue recording temperatures every minute for ten minutes.
- Have students graph the temperature data using graph paper and colored pencils. Have students write a caption for their graph that describes how the three different materials change in temperature over time.
- If several groups performed the experiment at the same time, have groups compare their graphs as they discuss findings.
Optional Extension:
Have students design and conduct their own research into the influence of surfaces on temperature comparing Earth surfaces that interest them (such as colored soils, dry and wet soils, grass, dry leaves, or different types of coverings such as plastic or aluminum foil). Have students compare the data with these new surfaces compared to the given surfaces (water, light soil, dark soil). Note that the data may not be comparable due to variations in experimental design, such as differences in light bulb temperature and height of the lamp.
Observations and Questions
- Which material absorbed more heat in the first ten minutes?
- Answer - Dark soil (dark-colored surfaces absorb most of the radiation instead of reflecting)
- Which material lost the most heat in the last ten minutes?
- Answer - Dark soil (the surface that absorbs the most heat, also emits the most heat)
- Imagine that it's summer and that the Sun is shining on the ocean and on a stretch of land.
- Which will heat up more during the day?
- Answer - The land heats up more than the ocean throughout the day. The darker-colored surface, the land, absorbs more of the Sun's radiation compared to the highly reflective surface of the ocean.
- Which will cool more slowly at night? Explain.
- Answer - The ocean will cool more slowly at night because surfaces that reflect most the radiation will also emit less radiation (analogous to Question 2)
- Which will heat up more during the day?
- Imagine three cities in the desert, all at about the same altitude and latitude. Which city would likely have the highest average summer air temperature and why?
- One city (A) is surrounded by a dark-colored rocky surface.
- Another city (B) is surrounded by a light-colored sandy surface.
- The third city (C) is built on the edge of a large man-made desert lake.
- Answer - City A will likely have the highest average air temperature because it has the darkest land surface. Darker land surfaces absorb higher amount of radiation and this absorption leads to an increase in the temperature.
- The Earth's surface tends to lose heat in winter. Which of the above cities would have the warmest average winter temperature? Why?
- Answer - City C (large man-made desert lake). A good reflector (desert lake) is a poor absorber (and a poor emitter) of radiation.
- Since the Sun is approximately 93 million miles from the Earth and space has no temperature, how do we get heat from the Sun?
- Answer - Refer back to the Earth's Energy Budget. The sunlight that makes it to the ground warms the Earth’s surface through absorption. The rest is reflected away by bright white clouds or ice or gets absorbed by the atmosphere.
Assessment
- In addition to student graphs and captions and participating in discussion, you may choose to have students respond to the discussion questions in their science notebook and submit it for evaluation or have students predict the heating and cooling curves of other types of materials and justify their predictions.
Modifications for Alternative Learners
- Do an oral assessment for students with limited reading and writing skills.
Background
Heat Transfer
Of the energy that reaches the Earth from the Sun, a small amount is absorbed by the atmosphere, a larger amount (about 30%) is reflected back to space by clouds and the Earth's surface, and most of it is absorbed at the planet surface and then released as heat.
Energy is transferred between the Earth's surface and the atmosphere in a variety of ways, including radiation, conduction, and convection. The graphic below uses a camp stove to summarize the various mechanisms of heat transfer. If you were standing next to the camp stove, you would be warmed by the radiation emitted by the gas flame. A portion of the radiant energy generated by the gas flame is absorbed by the frying pan and the pot of water. By the process of conduction, this energy is transferred through the pot and pan. If you reached for the metal handle of the frying pan without using a potholder, you would burn your fingers! As the temperature of the water at the bottom of the pot increases, this layer of water moves upward and is replaced by cool water descending from above. Thus convection currents that redistribute the newly acquired energy throughout the pot are established.
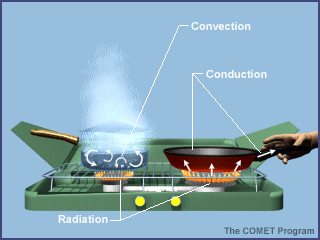
As in this simple example using a camp stove, the heating of the Earth's atmosphere involves radiation, conduction, and convection, all occurring simultaneously. A basic tenet of meteorology is that the Sun warms the ground and the ground warms the air. This activity focuses on radiation, the process by which the Sun warms the ground. Energy from the Sun is the driving force behind weather and climate.
Radiation
What do trees, snow, cars, horses, rocks, centipedes, oceans, the atmosphere, and you have in common? Each one is a source of radiation to some degree. Most of this radiation is invisible to humans but that does not make it any less real.
Radiation is the transfer of energy by electromagnetic wave motion. The transfer of energy from the Sun across nearly empty space is accomplished primarily by radiation. Radiation occurs without the involvement of a physical substance as the medium. The Sun emits many forms of electromagnetic radiation in varying quantities.
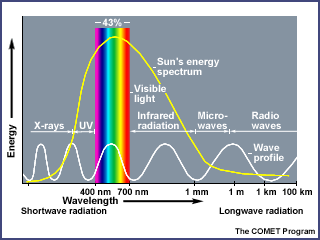
About 43% of the total radiant energy emitted from the Sun is in the visible parts of the spectrum. The bulk of the remainder lies in the near-infrared (49%) and ultraviolet section (7%). Less than 1% of solar radiation is emitted as x-rays, gamma waves, and radio waves.
A perfect radiating body emits energy in all possible wavelengths, but the wave energies are not emitted equally in all wavelengths; a spectrum will show a distinct maximum in energy at a particular wavelength depending upon the temperature of the radiating body. As the temperature increases, the maximum radiation occurs at shorter and shorter wavelengths. The hotter the radiating body, the shorter the wavelength of maximum radiation. For example, a very hot metal rod will emit visible radiation and produce a white glow. On cooling, it will emit more of its energy in longer wavelengths and will glow a reddish color. Eventually, no light will be given off, but if you place your hand near the rod, the infrared radiation will be detectable as heat.
The amount of energy absorbed by an object depends upon the following:
- The object's absorptivity, which, in the visible range of wavelengths, is a function of its color
- The intensity of the radiation striking the object
Every surface on Earth absorbs and reflects energy at varying degrees, based on its color and texture. Darker-colored objects absorb more visible radiation, whereas lighter-colored objects reflect more visible radiation.
Albedo
Albedo is the amount of energy reflected by a surface without being absorbed. An object with high albedo reflects the majority of the radiation while an object with low albedo reflects only a small amount of incoming radiation but absorbs the majority of the radiation.
Think about being outside and how different materials reflect or absorb the incoming radiation. White-colored surfaces such white paint reflect most of the radiation and therefore the albedo value is around 1. Dark-colored surfaces such as gravel and asphalt absorb most of the radiation and have low albedo values closer to 0. This also explains why darker-colored surfaces have higher temperatures. For example, in summer black asphalt roads can be scorching hot. As cities grow, and asphalt, concrete, and dark roofs replace the vegetation, these urban surfaces absorb—rather than reflect—the Sun's heat, causing surface temperatures and urban air temperatures to rise. This is known as the urban heat island effect.
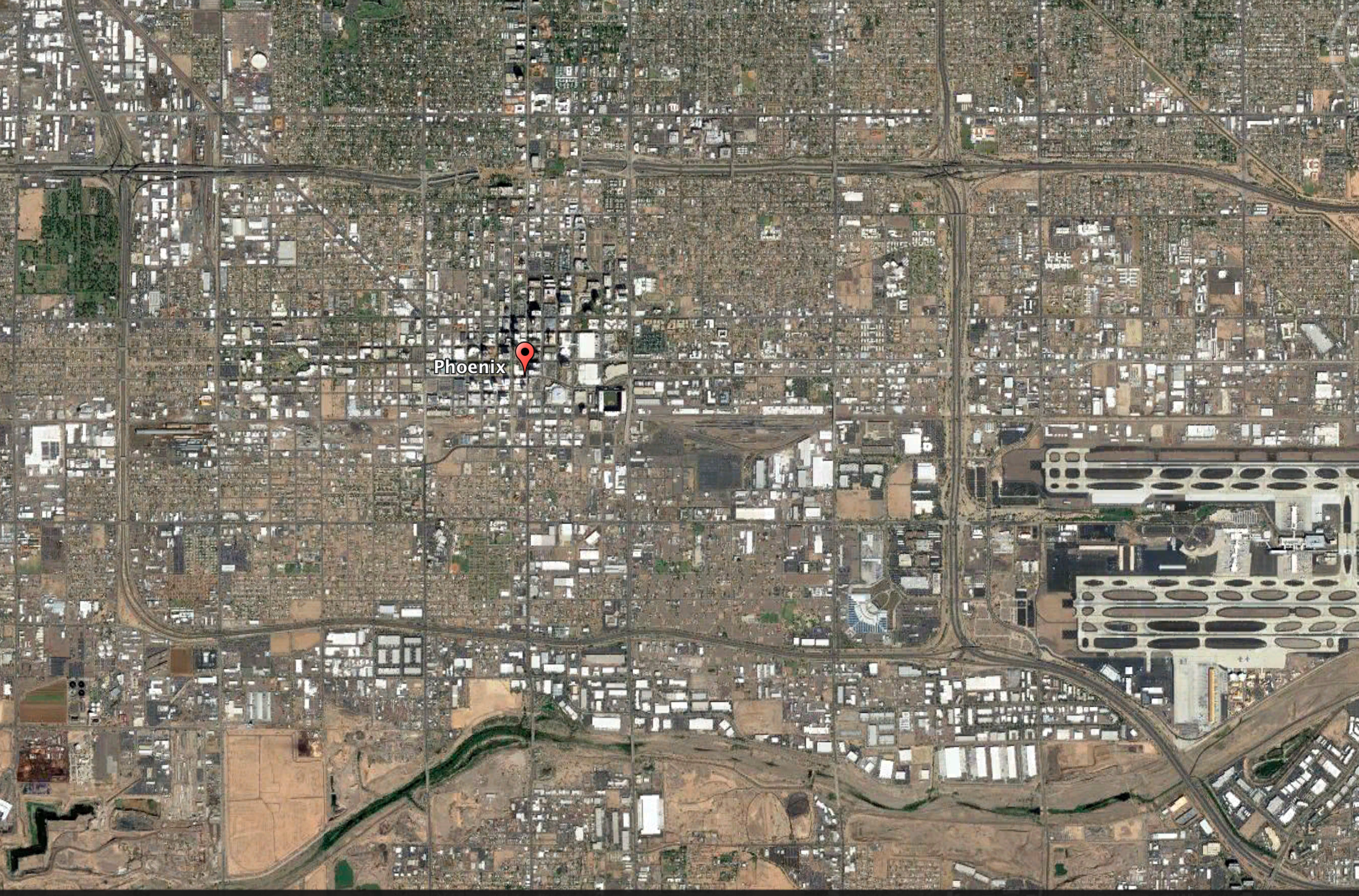
The city of Phoenix, Arizona, has made an effort to paint more roofs white to combat the urban heat island effect. The aerial view of Phoenix illustrates the different land surfaces such as water (river located at bottom of image), soil and rock (brown), vegetation (green), buildings with white roofs, roads, and an airport (lower right).
Credit: Google Earth