What do soda and the oceans have in common?
Students will use soda to explore how carbon dioxide is able to dissolve into liquid. They will learn about Henry's law, which describes how the solubility of gas into liquids is dependent on temperature, and develop hypotheses about how the amount of carbon dioxide in the atmosphere is affected by rising atmospheric and oceanic temperatures.
Learning Objectives
- Students will learn how atmospheric carbon dioxide gas (CO2) dissolves in water and that temperature is a key factor in the amount of gas that is dissolved in water (Henry's law).
- Students will relate Henry's law to Earth Systems science and climate change by considering how rising atmospheric and oceanic temperatures could impact ocean uptake of CO2.
Materials
- 1 unopened bottle of room temperature soda
- Cooled soda (40 degrees F)
- Warmed soda (100 degrees F - use a warm water bath)
- Small paper cups
- Stir sticks
- Student notebook or journal
- Access to the internet to watch videos
Directions
- Introduce the activity to students as a way to explore the significance of CO2 as greenhouse gas, ocean acidification, or even as a real life application of gas laws that explain how gas gets into a liquid. To determine prior knowledge about this topic, ask students questions such as:
- Have you heard that the oceans are carbon sinks?
- Have you ever wondered how carbon dioxide (CO2) from the atmosphere gets into the ocean?
- Tell students that the oceans play a dominant role in the uptake of atmospheric carbon that is created due to human activities, like burning of fossil fuels. Share that this process is hard to represent in climate models, and that scientists are trying to better predict how much of this extra carbon the ocean is able to absorb.
- Have students observe gas bubbles coming out of a bottle of soda and discuss the impact of pressure.
- Give each student group an unopened room temperature bottle of soda. Tell them that soda contains carbonated water which is what makes it bubbly. Tell them that we want to pay close attention to what happens when you open the bottle of soda.
- Have students write down observations in their notebook using the following questions as a guide:
- What happens when you open a bottle of soda?
- What happens if you slowly unscrew the top, wait a few seconds and then slowly screw the top back on?
- What makes the bubbles?
- Where was the CO2 before the bottle was opened?
- Why do you think bubbles rush out of the soda bottle when you open it?
- Pass out copies of the Student Activity Sheet to each student and give them time to read and watch the linked videos to learn about Henry's Law. Discuss the main points as a class:
- Gases can dissolve in liquid and is dependent on the chemical nature of the gas (solute) and the liquid (solvent), temperature, the partial pressure of the gas above the liquid.
- The scientific law that explains this is Henry's law.
- The dissolved carbon dioxide stays in the soda solution in the closed soda bottle, where the partial pressure in the space above the soda but below the cap was initially set high during bottling.
- High partial pressure means more carbon dioxide will go into solution in the liquid.
- When the bottle is opened the partial pressure above the liquid decreases allowing the carbon dioxide to come out of the solution.
- Have each group gather three equal-sized samples of soda in cups - one cup of room temperature soda, one warmed to around 100 degrees F, and the other cooled to around 40 degrees F. (The cooled soda can be kept in the refrigerator. The warmed soda can be kept in a warm water bath.)
- Students should make a hypothesis about the three different temperatures of soda to answer this question: How does temperature affect the amount of dissolved gas in a liquid?
- Have students make observations using their senses to determine which of the three samples is the flattest. Have them record observations in their notebooks.
- Visual observations of the bubbles can be made, they should see that the cold liquid has more bubbles or soluble gas and the warm liquid has few bubbles or soluble gasses.
- Students can stir using stir sticks to observe the amount of gas bubbles in each sample.
- Allow students to take sips of the soda if allergies are not a concern, or put a finger into the soda if sipping is not allowed. Each student should use their own individual paper cup for sipping. By sipping the soda, students can feel the differences between the amount of dissolved gasses in the warm, cold, and room temperature soda.
- Have each group discuss and share their conclusions about which sample has the most gas.
- Students should come to the conclusion that the warm soda has few bubbles because as temperature increases, solubility decreases. Conversely, as temperature decreases, solubility increases. This reasoning is supported by Henry's law. The warmer the liquid, the less gas it can retain, therefore the warm soda should have the least bubbles and the cold soda should have the most bubbles.
- Pose the following questions to have students consider how climate warming will affect the process of CO2 uptake in the ocean. (The ocean is able to absorb less CO2 as temperatures rise). This could be a small group discussion, a class discussion, or even have students write responses in their notebooks.
- How will the increase of anthropogenic (human caused) carbon dioxide change oceans all over the world?
- What are the implications to bringing heat and higher levels of CO2 to other deep ocean currents that are linked from the Southern Ocean?
- What other ocean ecosystems are affected by increased levels of CO2?
- Warmer temperatures may cause the ocean to absorb CO2 and other gases at a slower rate. How do you think that will affect the amount of CO2 and other gases in the atmosphere?
- What effect does decreased CO2 absorption by the ocean have on terrestrial ecosystems and human life?
- What are ways that anthropogenic CO2 can be reduced?
Assessment
Have students answer the assessment questions at the end of the Student Activity Sheet to assess their understanding of Henry's law. Recorded observations, hypotheses, and responses to discussion prompts are also opportunities for assessment.
(Answers: 1=C; 2=A; 3=B; 4=B; 5= all)
Background
Carbon dioxide molecule
Jacek FH, Licensed under CC BY-SA 3.0 via Commons
In this activity, students explore how atmospheric carbon dioxide (CO2) gas gets into and out of the ocean. This is important because the ocean currently absorbs much of the carbon dioxide that we add to the atmosphere. This is not enough to mitigate all of the anthropogenic carbon dioxide, but it has reduced the amount currently in the atmosphere. However this uptake of CO2 by the oceans effects the pH level, which among other things causes coral bleaching. The amount of the gas that is able to be taken up by the ocean is regulated by Henry's law.
- Henry’s law: At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
Within a carbon dioxide molecule (one oxygen atom and two carbon atoms), the atoms share electrons unevenly. This means that the oxygen side of the molecule has a slight negative charge, making it is a (weak) non-polar molecule.
Water (H2O) is made up of two hydrogen atoms and one oxygen atom. Again, the atoms share electron, but not evenly. This uneven sharing of electrons give water molecules a slight positive charge near the two hydrogen atoms and a slight negative charge near the oxygen atom, which is called a polar molecule.
To dissolve in the water, the CO2 molecule must pass through the air-water surface, where the CO2 molecule gains an outer shell of the H2O molecule. The positive charge, or electron-rich, area of the water molecule attracts the negative charge, or electron-deficient, is of the carbon dioxide molecule allowing it to go into solution. This process transfers the molecule from a gas into a liquid.
Water molecule
Dbc334; Jynto, Public domain, via Commons
- Impact of Pressure: Air contains a mixture of gases, each of which has a partial pressure that contributes to the total sum of the pressure. The higher the concentration of a gas outside of the liquid, or partial pressure, the more gas of that gas will be able to go into solution.
- Impact of Temperature: Gas solubility is temperature-dependent. Gas can dissolve more readily in colder liquids. At the same pressure, the colder temperature allows a gas to stay in the solution longer.
How is this applied to the ocean and carbon dioxide?
The ocean temperature impacts the solubility of gases such as carbon dioxide. Colder ocean water can dissolve more CO2 than warm ocean water. So in theory, colder water in the polar regions can take up more CO2 than warmer equatorial waters.
Scientists are concerned that increased CO2 in the atmosphere could create a positive feedback loop, a cycle in which the effects of a change in a system increase the magnitude of the change. For example, if ocean temperatures warm, CO2 could be released from solution, increasing the atmospheric concentrations leading to further heat-trapping mechanisms, or at least slowing the rate of uptake by the oceans. The cycle continues to warm the atmosphere and the ocean.
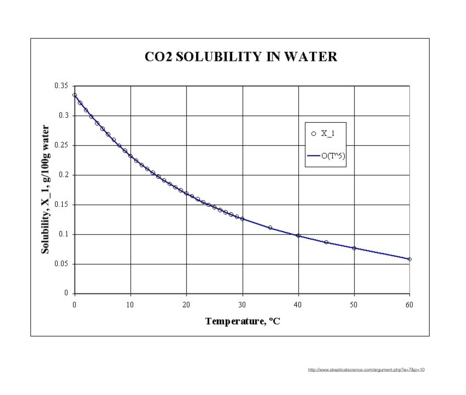
Solubility, X_1, of CO2 in water. Handbook of Chemistry & Physics, 34th ed., 1953, Solubility of Gases in Water, p. 1532. The curve is the best–fit, fifth-order by the author.
How does soda relate to Henry's law and carbon dioxide uptake by the ocean?
After learning about Henry's law, students should be prepared to develop a hypothesis about the state parameters of each of the three temperature sodas, hopefully recognizing that the warm soda will retain the least amount of fizz and that the cold bottle will retain the most fizz (and thus, the most CO2). Students should come to the conclusion that the warm soda has few bubbles because as Henry’s Law indicates, as temperature increases, solubility decreases, and conversely, as temperature decreases solubility increases.