Whole Body Ozone Chemistry
In this activity, students will play the roles of various atoms and molecules to help them better understand the formation and destruction of ozone in the stratosphere.
Learning Objectives
- Students will understand how ozone is formed in the Earth's stratosphere and will be able to explain the importance of stratospheric ozone.
- Students will be able to explain how ozone is destroyed in the stratosphere.
- Students will understand that some chemicals can speed up the breakdown of ozone in the atmosphere.
- Students will be able to explain why chlorofluorocarbons (CFCs) are destructive to the ozone layer.
Materials
- 8 1/2 by 11 sheets of paper or cardboard
- Hole punch
- Magic markers
- String
- Flashlight
- Clear red and blue plastic sheets to cover flashlight lens
- String (optional)
Directions
Note: As written, this activity requires that students hold hands. Younger students may not have any problems with this, however, the self-consciousness of adolescents may hinder the spontaneous movement and physical contact required for this activity. If you think this will be problematic in your classroom, cut 12-inch lengths of string for the students to hold to make the 'bonds.'
Note: care must be taken with the younger grades to make the atomic concepts simple and clear. You may wish to eliminate the more complex CFC reactions, for example.
This activity should be done a step at a time, being sure the students understand the analogy. Otherwise, the analogy may be confusing or more difficult to understand than the concepts being illustrated. It is essential to stop and discuss after each section.
Part 1: Modeling Oxygen in the Earth's Atmosphere
- Let 5 or 6 pairs of students represent oxygen molecules. Each student should construct a sign using a piece of paper, writing a large O on it and attaching a string to go around their neck, indicating they are oxygen atoms.
- Students in each pair should hold hands to simulate the bonding between the atoms of oxygen in each molecule. Have these pairs of students move about in a cleared area in the classroom to simulate molecular motion. It is appropriate for them to bounce off a wall or collide with each other as they move about. After moving about for a minute or so, stop to discuss what has been demonstrated.
- Discussion:
- How are the moving pairs of students similar to what occurs in the air in the room? (Oxygen in the air exists as two atoms to each molecule, and, like all air molecules, oxygen is constantly in motion.)
- How is it different? (Obviously, the pair of students is much larger than one oxygen molecule. In addition, air has other gases—nitrogen, carbon dioxide, and other trace gases.)
- What could be done to make the analogy better? (Some suggestions might include having other students act as nitrogen atoms, carbon dioxide molecules, etc. To make it more realistic, how many nitrogen molecules () should be used for each oxygen molecule ()? About four, since air contains about 80% nitrogen and 20% oxygen.)
- What is oxygen called if it has two atoms per molecule? (Diatomic oxygen also known as molecular oxygen. A single O atom is known as atomic oxygen.)
Part 2: Simulating the Formation of Ozone in the Stratosphere
- Repeat the steps under modeling the earth's oxygen, but this time darken or dim the lights in the room.
- Add a student who, with a flashlight, simulates solar radiation. Place a clear blue plastic sheet over the lens of the flashlight to represent the ultraviolet short wavelengths that are involved in the breakup of diatomic oxygen.
- Let pairs of students representing oxygen begin their motion as before. When the student with the flashlight shines the light on a pair of students, the bond between them breaks, and students let go of their partner.
- As the motion continues, these single atoms of oxygen move around until they bump into a pair of oxygen atoms. Each of the single oxygen atoms combines with the pair they bump into, forming a group of three oxygen atoms. These three students hold hands, representing a molecule of ozone.
- Discussion:
- How is this simulation similar to the way ozone is formed in the stratosphere? (UV light breaks the bonds on oxygen molecules, and the free oxygen atom combines with other oxygen molecules to produce ozone.)
- What is oxygen with three atoms per molecule called? (ozone)
- How many molecules of ozone can be formed by the breakup of one molecule of diatomic oxygen by ultraviolet light? (2)
- Why is ozone formed this way in the stratosphere and not in the air near the earth's surface? (Much more ultraviolet light exists in the stratosphere than near the earth's surface.)
Part 3: Demonstrating How Ozone Breaks Down in the Stratosphere
- Have several groups of three students, each representing ozone, move about the room. Pairs of students representing diatomic oxygen can be added as a touch of realism.
- This time the lens of the flashlight should be covered with clear red plastic to represent UV light of a longer wavelength.
- When this light is used to illuminate an ozone molecule, the ozone breaks up to form a diatomic molecule (a pair of students) and an oxygen atom (single student).
- This process is repeated by shining the light on a second ozone molecule, producing another pair of oxygen atoms and another single oxygen atom.
- The two single oxygen atoms should then combine to form a pair of atoms, or a molecule of diatomic oxygen.
- Discussion:
- How many molecules of diatomic oxygen are formed from the breakup of two molecules of ozone? (3)
- How is the breakup of ozone in the stratosphere similar to its formation there? (Both the formation and breakup of ozone involve UV light, but different wavelengths.)
Part 4: An Example of a Chemical that Speeds up the Breakdown of Ozone
-
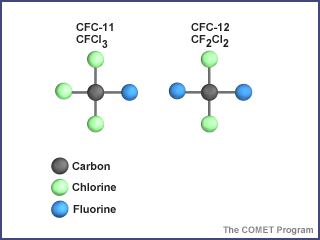
The molecular structure of common CFCs
Credit: UCAR/COMETOf all the chemicals involved in the breakdown of stratospheric ozone, none have received more attention than the chlorofluorocarbons, or CFCs. The two most common are CFC-11 () and CFC-12 (). These compounds can be modeled by letting students represent atoms of carbon (C), chlorine (Cl), and fluorine (F). For example, a molecule of CFC-11 would be composed of one student representing a carbon atom, another representing a fluorine atom, and three students representing three chlorine atoms. The students should hold hands to demonstrate how atoms are bonded in a molecule.
- Discussion:
- The CFCs are inert, that is, they do not react with other materials under most conditions. How can this be demonstrated using groups of students to represent atoms of different elements? (The CFCs can move around together, but students should lock elbows, showing that the bonds of these molecules do not break apart easily.)
- The CFCs that enter the atmosphere at the earth's surface have found their way into the stratosphere. How can this be demonstrated using students to play the role of various gases in the air? (The CFCs can gradually move from the place designated in the classroom as the earth's surface to the place designated as the stratosphere. More ozone molecules should be in the stratosphere. The student with the flashlight representing UV should be in the place designated as the stratosphere.)
Part 5: The Role of Chlorine in the Breakdown of Ozone in the Stratosphere
- UV light breaks down CFCs in the stratosphere, releasing chlorine atoms. This can be demonstrated by having a student with a flashlight shine a light on a group of students representing a molecule of CFC-11 or CFC-12. Let one student representing a freed chlorine atom move amidst groups of students representing ozone. The chlorine is involved in the breakdown of ozone as follows:
- Cl + ----> ClO +
- ClO + O ----> Cl +
- A student representing chlorine pulls an oxygen atom away from an ozone molecule to form chloride oxide (ClO).
- The two students representing ClO react with an oxygen atom.
- The two students representing oxygen combine to form an oxygen molecule.
- The student representing chlorine is then free to attack another molecule of oxygen.
- Repeat these steps several times to show the chain reaction.
- Discussion:
- What is a catalyst? (A chemical that promotes a chemical reaction but is not used up in the reaction.)
- Does the chlorine act as a catalyst in this reaction? (Yes)
- Why is the involvement of chlorine in the breakdown of ozone called a chain reaction? (Chlorine can cause the breakdown of many ozone molecules and the chlorine is not altered or destroyed.)
Assessment
Because this is a complex, multistep simulation, it would be difficult for the teacher to informally observe or question each student during the activities. We suggest instead that students keep a log of the discussion questions and answers as they go, to be turned in and evaluated by the teacher.
Draw an unlabeled set of simple "ball and stick" molecular pictures on overheads illustrating each of the activities done by the students. Have students copy the overhead drawings and label each molecule and process.
Provide gumdrops or clay and toothpicks for students to build the molecular models.
Modifications for Alternative Learners
The kinesthetic nature of the lesson will be easily followed by English Language Limited students, but the connection to the molecular processes may be difficult. Use overhead illustrations liberally to connect the student activities to the processes, rather than relying only on voice.
Students with physical limitations could be given gumdrops or clay and toothpicks to simulate molecular models.
Background
Ozone, a molecule containing three oxygen atoms, is made when UV light breaks the bonds of oxygen molecules containing two oxygen atoms in the stratosphere. The single oxygen atom is highly reactive and bonds with another oxygen molecule creating ozone.
By having students play the roles of various atoms and molecules, ideas of basic chemistry in the atmosphere are made more concrete. For example, pairs of students can represent diatomic oxygen while a trio is required for ozone. This illustrates chemical reactions involved in the photochemistry of ozone production and destruction, along with a catalyst that affects the rate of the reaction.
Learn more: