Ozone
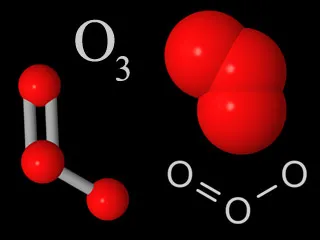
Four representations chemists use for ozone molecules. Each ozone molecule has three oxygen atoms, unlike normal molecular oxygen which only has two atoms per molecule.
UCAR
Ozone is a special kind of oxygen molecule. Normal oxygen molecules (O2), the kind we need to breathe, have two oxygen atoms. Ozone molecules (O3) have three oxygen atoms.
Ozone is found in two different layers in Earth's atmosphere. "Bad" ozone is found in the troposphere, the layer nearest the ground. Tropospheric ozone is a harmful pollutant which forms when sunlight alters various chemicals emitted by humans. "Good" ozone forms in the stratosphere, the next higher layer where some jet planes fly. Stratospheric ozone forms naturally and helps shield us from ultraviolet (UV) radiation in sunlight that can cause sunburn and skin cancer.
Ozone in the stratosphere forms when a photon of ultraviolet "light" from the Sun strikes a normal oxygen molecule, breaking it apart. One of the atoms freed by this photodissociation process attaches itself to another oxygen molecule, converting it into ozone. Part of the stratosphere is sometimes called the "ozone layer".
In the troposphere, a range of chemical reactions can create ozone. Tropospheric ozone is formed when sunlight strikes various human-created pollutants, including nitrogen oxides, carbon monoxide, and hydrocarbons. Ozone can irritate the throat and lungs and causes a burning sensation in eyes. Ozone also harms plants and damages some types of materials, especially objects made of rubber.
Ozone in the stratosphere protects us from ultraviolet radiation in sunlight. The ozone layer is sort of like sunscreen for planet Earth. It absorbs most of the incoming UV "light" before it reaches the ground. The ozone molecules which absorb UV radiation later re-radiate the energy as heat, warming the stratosphere.
Various chemicals that humans release into the atmosphere can destroy ozone in the stratosphere. This has caused a thinning of the ozone layer in recent years, and even holes in the ozone layer over Earth's poles.
Ozone molecules are much less stable than regular (O2) oxygen molecules. They are very prone to react chemically with other substances. Ozone is also a type of greenhouse gas. Scientists think it is responsible for about 3-7% of the greenhouse effect on Earth.
© 2014 UCAR with portions adapted from Windows to the Universe (© 2009 NESTA)