Ozone in the Troposphere
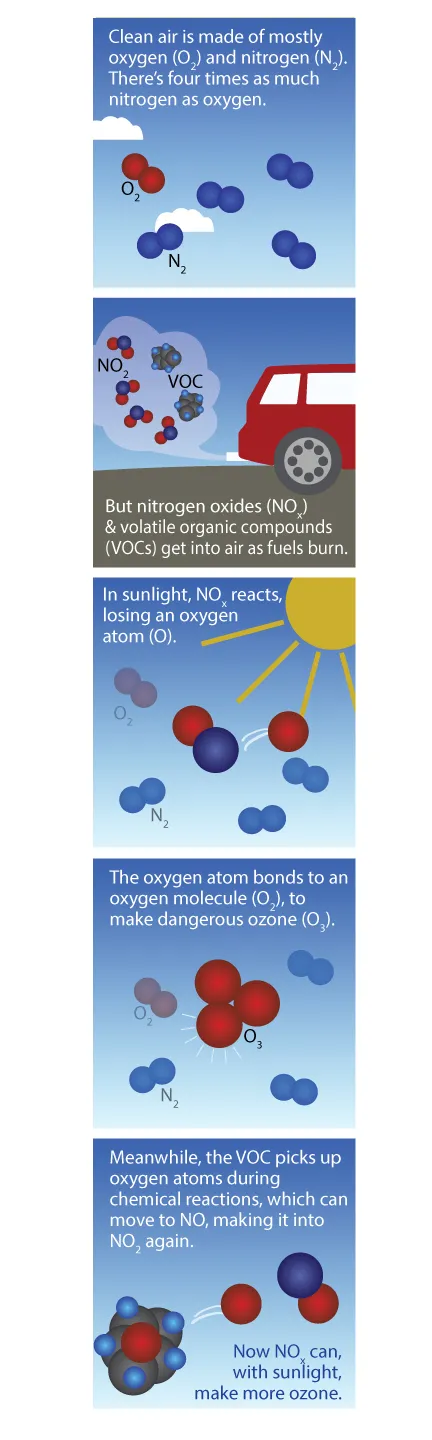
How ground level ozone is formed in the air
UCAR/L.S. Gardiner
In the stratosphere, ozone molecules play an important role - absorbing ultraviolet radiation from the Sun and shielding Earth from dangerous rays. But in the troposphere, near ground-level, ozone molecules are both air pollutants, threatening the health of living things, and greenhouse gases, trapping heat and contributing to climate change.
A small amount of ozone does occur naturally at ground level. Plants and soil release some. Some migrates down from the stratosphere. But neither of these sources contributes enough ozone to be considered a threat to the health of humans or the environment. Most of the ozone that is found near the ground comes from vehicle exhaust and emissions from factories, power plants, and refineries. Since 1900, the amount of ozone near the Earth's surface has more than doubled due to more automobiles and industry.
Unlike most other air pollutants, ozone is not directly emitted into the air. Tropospheric ozone is formed by the interaction of sunlight, particularly ultraviolet light, with hydrocarbons and nitrogen oxides, which are emitted by automobile tailpipes and smokestacks. In urban areas, high ozone levels usually occur during warm summer months. Typically, ozone levels reach their peak in mid to late afternoon, after exhaust fumes from morning rush hour have had time to react in sunlight. A hot, sunny, still day is the perfect environment for the production of ozone pollution. At the end of the day, as the Sun starts to set, the production of ozone begins to subside. To form, ozone needs sunshine to fuel the chemical reaction.
When ozone pollution reaches high levels, pollution alerts are issued urging people with respiratory problems to take extra precautions or to remain indoors. When it’s inhaled, ozone can damage lung tissues. Ozone is harmful to all types of cells. It can impair an athlete's performance, create more frequent attacks for individuals with asthma, cause eye irritation, chest pain, coughing, nausea, headaches and chest congestion. It can worsen heart disease, bronchitis, and emphysema.
Ozone also damages materials like rubber, textile dyes, fibers, and certain paints. These materials can be weakened or degraded by exposure to ozone. Some elastic materials can become brittle and crack, while paints and fabric dyes may fade more quickly.
What can we do to decrease the production of ozone in the troposphere? Choosing public transportation, walking, or biking instead of traveling in cars is a good step. If you wait until evening to refuel your car or mow your lawn, it's unlikely that the pollutants released will become ozone. And, on a larger scale, you can look for energy sources that don’t emit the pollution that leads to ozone. Check with your utility company to find out where your energy comes from.
Check out the EPA’s Actions You Can Take to Reduce Air Pollution for more tips to reduce air pollution.