Air Pollution: How We're Changing the Air

Factories like this oil refinery are a major source of air pollution.
UCAR
Air pollution is created when harmful substances, in the form of gases, liquids, or solids, enter the air. There are natural processes that create air pollution— such as sulfur and chlorine gases from volcanic activity, smoke and ash from wildfires, dust storms, and biological decay — but most pollution enters the air from human-made (anthropogenic) sources.
Most human-made air pollution comes from burning fossil fuels for transportation, electricity, and industry. Common pollutants produced by engines that burn fossil fuels are carbon dioxide, nitrogen oxides, sulfur dioxide, volatile organic compounds (VOCs), and particulates. Stoves, incinerators, and open burning produce carbon monoxide and carbon dioxide, as well as particulates.
We also create chemicals that would not naturally occur in the atmosphere. Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs), which are used as refrigerants, are examples of pollutants that only come from human activity. Other sources of CFCs include fumes from aerosol sprays, paint, varnish, and solvents.
Six Types of Human-Made Air Pollutants
The Environmental Protection Agency (EPA) Air Quality Index (AQI) is based on the measurements of six pollutants: particulate matter (PM), nitrogen dioxide (NO2), ozone (O3), sulfur dioxide (SO2), lead (Pb), and carbon monoxide (CO).
Particulate Matter (PM)
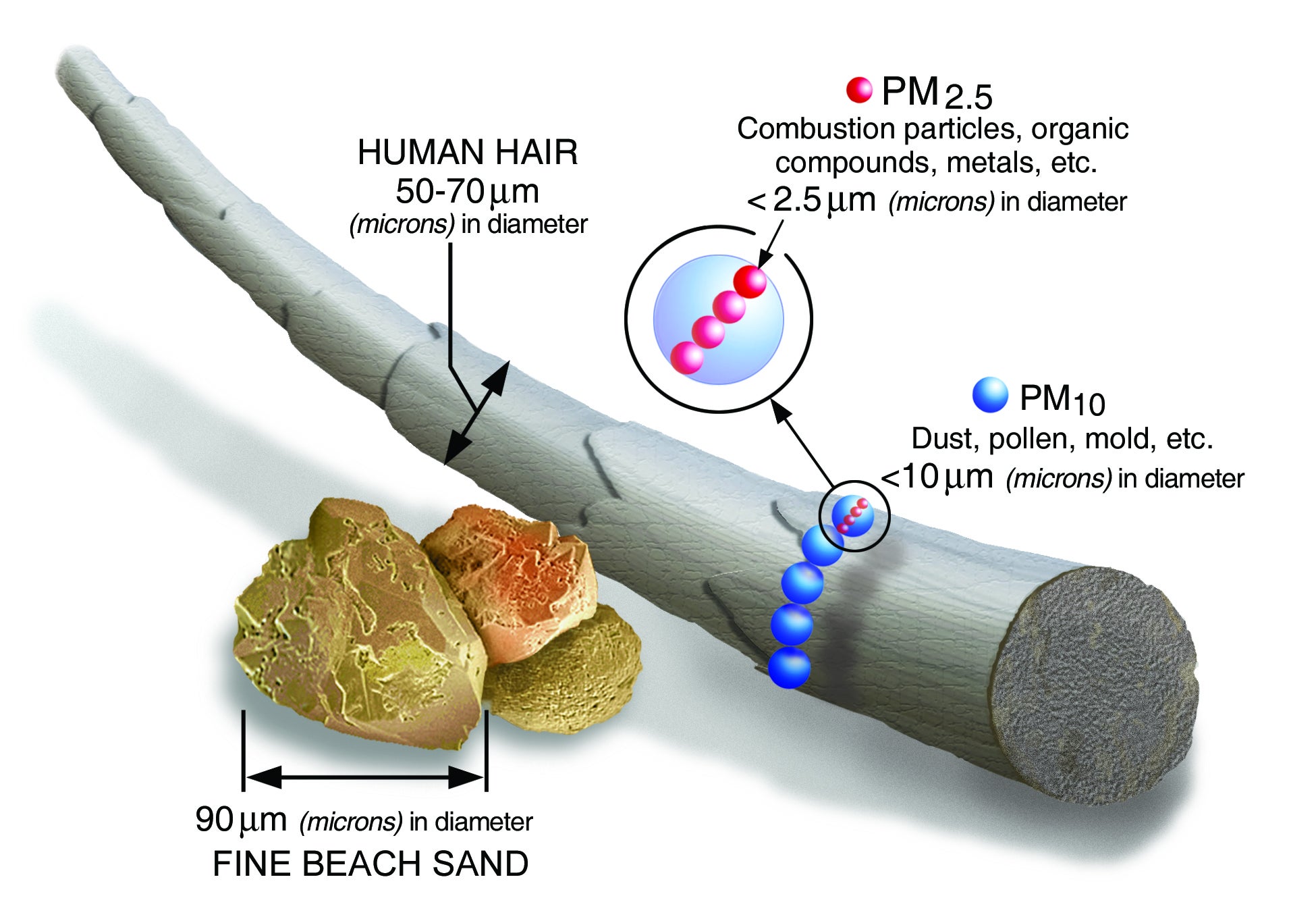
How small is 2.5 micrometers? The average human hair is between 50 and 70 micrometers. Fine particulate matter is so tiny that it enters the lungs and even the bloodstream.
EPA
Particulate matter, also called aerosols or particle pollution, is composed of a large variety of chemicals. Some particles are primary pollutants that come directly from smokestacks, construction sites, fires, or volcanoes. However, most are secondary pollutants that form as the result of chemical reactions in the atmosphere due to emissions from power plants, factories, and vehicles.
Particulate matter is classified by size:
- PM10 is coarse particles with a diameter of 2.5 to 10 micrometers, such as dust, dirt, pollen, and mold.
- PM2.5 is fine particles with a diameter of 2.5 micrometers or less, such as soot, smoke, organic compounds, and metals.
Nitrogen Dioxide
Vehicle exhaust is the largest source of nitrogen dioxide pollution in the atmosphere, but it is also formed by factories and power plants, and naturally by lightning strikes, volcanoes, and during the decomposition of organic matter. Nitrogen dioxide causes the characteristic reddish-brown color of smog and reacts in the presence of sunlight to produce harmful ozone.
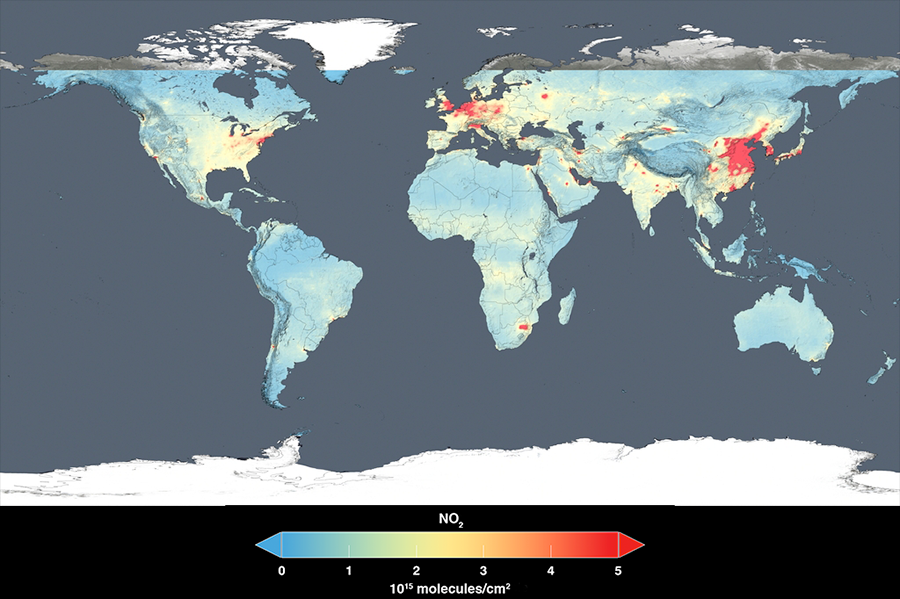
This global map shows the 2014 average concentration of nitrogen dioxide in the troposphere detected by the Ozone Monitoring Instrument aboard the Aura Satellite. Nitrogen dioxide hotspots, visible here over most major cities and developing nations, are used as an indicator for general air quality.
NASA Goddard Space Flight Center
Ozone (O3)
Though ozone naturally exists within the stratosphere, it does not typically occur at the surface. Ozone in the troposphere is a secondary air pollutant created from chemical reactions with other air pollutants. It forms when nitrogen oxides from tailpipes and smokestacks react with VOCs in the presence of sunlight. Tropospheric ozone, also called ground-level ozone, is most common in urban areas and is often measured at high levels in the summer and during the warmest times of the day.
One common type of secondary pollution that contains ozone is smog. Smog is a smelly, toxic haze composed of ground-level ozone, particulate matter, and a variety of other chemicals. Smog is an example of how primary air pollution from human activity can lead to even more harmful secondary pollution.
This panel shows how a secondary pollutant is formed. Primary pollutants (nitric oxide and VOCs) from vehicle emissions react with sunlight to create a new pollutant (ozone) in the atmosphere.
UCAR
Sulfur Dioxide (SO2)
The smell associated with burnt matches comes from sulfur dioxide. Almost all sulfur dioxide in the atmosphere is the result of human activity, though volcanoes are also a natural source. Coal, oil, and gas often contain sulfur, as do some mineral ores. The burning of any of these sulfur-containing materials, during industrial processing or the generation of electricity, releases toxic sulfur dioxide and sulfur trioxide, together called sulfur oxides, into the atmosphere. When mixed with water droplets suspended in the air, sulfur dioxide forms sulfuric acid, which is a component of acid rain.
Lead (Pb)
Lead is a heavy metal found naturally underground. It enters the air through ore and metals processing, and through the burning of leaded fuel for aircraft and vehicles. The EPA’s requirement to remove lead from “on-road” vehicle gasoline resulted in a 98% decrease in lead levels in the air between 1980 and 2014 (leaded fuels are still allowed for race cars, farm equipment, and propeller aircraft).
Carbon Monoxide (CO)
If something is burning, carbon monoxide is likely being released. Carbon monoxide is a poisonous gas that is created when carbon is burned. Most of the carbon monoxide pollution comes from burning fossil fuels in vehicles, factories, and power plants, but another major source is from burning wood or crop waste. Carbon monoxide is released from volcanoes and forest fires as well. Secondary pollutants like ozone and carbon dioxide (CO2), a greenhouse gas, come from carbon monoxide.
Greenhouse Gas Pollution
The burning of fossil fuels for electricity, heat, and transportation is increasing the amount of greenhouse gases such as carbon dioxide (CO2), nitrous oxide (N2O), and methane (CH4) in our atmosphere. Small amounts of these gases in the atmosphere are safe to breathe but are also dangerous because they are changing Earth’s climate through a process called the greenhouse effect. Land-use changes due to farming and forestry also lead to an increase in greenhouse gas emissions. For example, livestock and their waste release methane, and cutting down trees means less carbon dioxide is removed from the air. The shift from coal to natural gas as an energy source and the increased use of renewable energy is helping to slow carbon dioxide emissions, but overall, greenhouse gas emissions remain high. As long as greenhouse gas emissions remain high, global average temperatures will continue to rise.
Heavy traffic in cities results in pollution from motor vehicle exhaust. These pollutants contribute to global climate change as greenhouse gases trap heat within the Earth’s atmosphere.
UCAR/MIRAGE
Indoor Pollutants
Formaldehyde, asbestos, radon gas, and mold are common harmful indoor air pollutants. Many products used in buildings, such as foam insulation, carpet, and furniture glues, give off high levels of volatile organic compounds (VOCs). Paints, varnishes, and cleaning materials also contain VOCs. Smoking indoors adds many pollutants, including cancer-causing chemicals, to the air. If you burn natural gas or wood to heat your home, be sure to monitor for elevated carbon monoxide levels.
Because we typically spend many hours indoors, and because indoor pollutants are confined within a small area, the level of exposure needed to cause harm is much lower than for outdoor pollutants. To reduce health risks from indoor pollutants, limit or eliminate the use of products that give off VOCs within your home. Ensure that homes and buildings have adequate ventilation and open doors or windows when using chemicals or introducing materials that contain VOCs to your indoor environment. Visit the EPA’s website to learn more about indoor air quality.