Sulfur Oxides
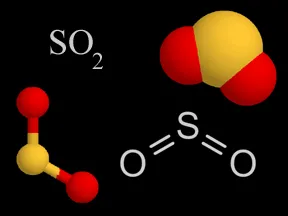
Above are four different ways chemists represent sulfur dioxide. In the models, the yellow atom is sulfur and the red atoms are oxygen.
UCAR
Sulfur oxides are a group of molecules made of sulfur and oxygen atoms, such as sulfur dioxide (SO2) and sulfur trioxide (SO3). Sulfur oxides are pollutants that contribute to the formation of acid rain, as well as particulate pollution. Some are released into Earth’s atmosphere by natural sources, but most are the result of human activities.
Some sulfur oxides are gases, and some are liquids or solid particles. Sulfur dioxide is the most dangerous of the sulfur oxides. It is a colorless gas that smells like burnt matches. Sulfur trioxide is often a colorless or white solid that creates white fumes in the air and has strong reactions with water. Both sulfur dioxide and sulfur trioxide react to form sulfuric acid, which is toxic to living tissue and is the main component of acid rain.
Sources of Sulfur Oxides
Volcanoes are a natural source of sulfur oxides in the atmosphere, but 99% of the sulfur dioxide in the atmosphere comes from human activity, such as burning coal, oil, and gas to make electricity and heat. When coal and oil burn, the sulfur in them combines with oxygen in the air to make sulfur oxides. Processing mineral ores that contain sulfur and industrial burning of fossil fuels are also sources of sulfur oxides in the atmosphere.
Sulfur Oxides as Pollutants
Sulfur oxides pollute the air. They are harmful to your lungs and make it difficult to breathe. Sulfur dioxide forms sulfur particles, which, if inhaled regularly, can cause asthma and bronchitis. Sulfur trioxide vapors are toxic if inhaled and cause burns to the skin and organs. Sulfur oxides also combine with water droplets in the air to make sulfuric acid, which is part of acid rain. Acid rain is bad for plants and fish and other living things. Sulfur oxides combine with other molecules to form particles that contribute to particulate pollution, creating hazy skies with reduced visibility.
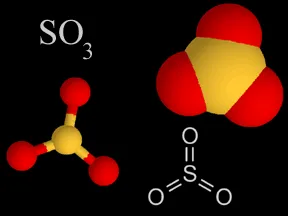
Above are four different ways chemists represent sulfur trioxide. In the models, the yellow atom is sulfur and the red atoms are oxygen.
UCAR
Use of Sulfur Oxides
Fuels contain sulfur oxides, but recent reductions in the allowable amount of sulfur in regular and diesel fuel, along with tighter controls on vehicle emissions, have led to a decrease in sulfur oxide pollution. Sulfur dioxide is used as a food preservative, especially for dried fruits. It is also used as a refrigerant, a disinfectant, and as a bleaching agent. Sulfur trioxide is used to manufacture chemicals and in explosives.
© 2020 UCAR with portions adapted from Windows to the Universe (© 2014 NESTA)