What's in the Air?
Three-fourths of all air resides in the troposphere, the lowest layer of the Earth’s atmosphere. Air is a mixture of gases, most of which are naturally occurring. Air also contains a significant amount of human-made air pollutants, including some that are not safe to breathe and some that are causing the climate to warm. The troposphere also contains water in all three phases (liquid, solid, and gas) as well as solid particles called aerosols.
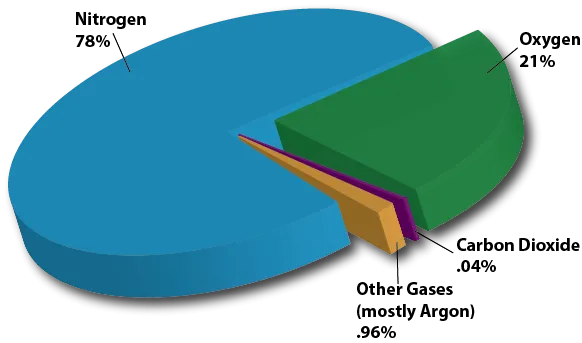
The dry composition of the atmosphere is mostly nitrogen and oxygen. It also contains fractional amounts of argon and carbon dioxide and trace amounts of other gases, such as helium, neon, methane, krypton, and hydrogen (NASA).
UCAR
Gases in the Air
The most abundant naturally occurring gas is nitrogen (N2), which makes up about 78% of air. Oxygen (O2) is the second most abundant gas at about 21%. The inert gas argon (Ar) is the third most abundant gas at 0.93%. There are also trace amounts of carbon dioxide (CO2), neon (Ne), helium (He), methane (CH4), krypton (Kr), hydrogen (H2), nitrous oxide (NO), xenon (Xe), ozone (O3), iodine (I2), carbon monoxide (CO), and ammonia (NH3) in the atmosphere.
Water Vapor
Due to the water cycle, the amount of water in the air is constantly changing. The lower troposphere can contain up to 4% water vapor (H2O) in areas near the tropics, while the poles contain only trace amounts of water vapor. The concentration of water vapor decreases drastically with altitude. The upper troposphere has less water vapor than air near the surface, the stratosphere and mesosphere have almost no water vapor, and the thermosphere contains none at all.
Aerosols
Air also contains tiny solid particles called aerosols, such as dust, sea salt, and ash from erupting volcanoes or forest fires. Many of these particles are so small that they are microscopic. Others are large enough to see. Aerosols affect climate by helping clouds form and shading the planet by scattering or absorbing sunlight. In the last century, manufacturing and widespread use of combustion engines have increased the number of aerosols in the atmosphere as particulate matter spews from smokestacks and exhaust pipes. Burning wood and other materials also add particles to the air.
Atmospheric Chemistry
Like everything on Earth, the air is made of chemicals. The chemicals in the air often combine with each other, or with other chemicals from the Earth’s surface, through chemical reactions. Many of these chemical reactions help maintain healthy natural environments and are vital for plants and animals. Nitrogen gas in the atmosphere does almost nothing, but nitrogen elsewhere on Earth is essential for life. Through the nitrogen cycle, nitrogen makes its way into soil and water, binds with other elements, and can be used by living things. Oxygen from the atmosphere causes oxidation reactions that help break down matter and release nutrients into soils, and is used by humans and animals in cellular respiration.
Atmospheric chemistry in the troposphere is also influenced by human-made chemicals that can negatively impact human health and the environment. For example:
- Vehicle exhaust contains nitrogen dioxide, as well as other polluting chemicals such as carbon monoxide and sulfur dioxide. Nitrogen dioxide reacts with atmospheric oxygen to form tropospheric ozone which is hazardous to plant and animal cells.
- Smog, which is mainly made of ozone and particulate carbon (soot) emitted by coal-burning power plants, causes damage to the lungs of humans and animals.
- Factories that burn fossil fuels also release sulfur and nitrogen dioxides, which combine with water in the atmosphere to make acid rain. Acid rain causes damage to natural and human-made environments.
Chemistry of the Air
The table below lists the major gas components and their role in the atmosphere. Click on each molecule name to learn more about them.
| Gas | Chemical & Molecular Structure |
Role in the Atmosphere |
|---|---|---|
| Nitrogen | 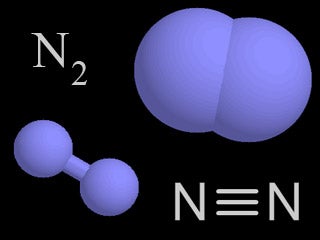 |
78% of the air in the atmosphere is nitrogen. Nitrogen is transferred to plants, animals, and the environment through the nitrogen cycle. |
| Nitrogen Oxides | 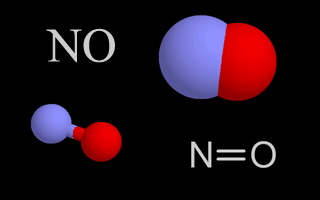 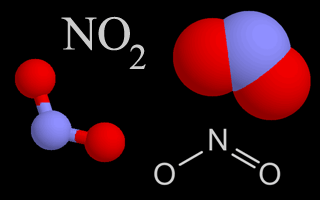 |
Nitrogen oxides are air pollutants that contribute to the formation of ozone. They also create nitric acid when they mix with water droplets in the air, which is part of acid rain. |
| Oxygen | 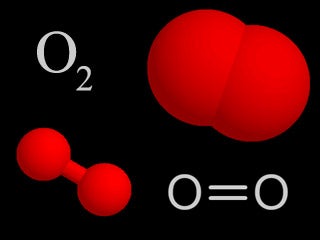 |
Oxygen makes up 21% of the atmosphere. It is highly reactive and forms compounds with numerous other chemicals, and is necessary for respiration in living things. |
| Ozone | 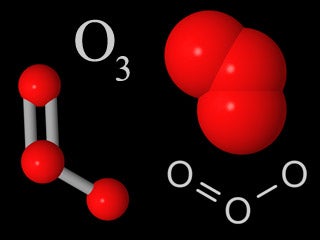 |
Ozone in the troposphere is a human-made pollutant. Ozone in the stratosphere forms the ozone layer, which is crucial for the survival of life at the Earth’s surface. |
| Argon |  |
Argon makes up about 1% of the atmosphere and comes mostly from the decay of potassium in the Earth’s crust. It is an inert gas, which means that it does not react with other chemicals. |
| Water Vapor | 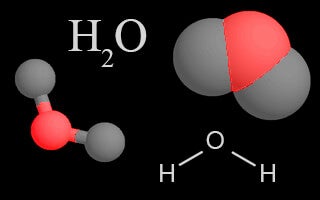 |
Water is cycled through all of Earth’s systems in each of its three phases: solid, liquid, or gas. Water vapor in the atmosphere is a greenhouse gas due to its heat-trapping ability. |
| Carbon Dioxide | 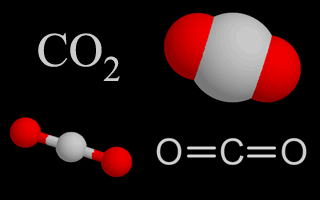 |
Carbon dioxide naturally composes about 0.03% of the atmosphere, but the amount is increasing due to the burning of fossil fuels. Plants and eubacteria use carbon dioxide during photosynthesis. Humans, other animals, and plants add it to the air through respiration. Carbon dioxide is a heat-trapping greenhouse gas. |
| Carbon Monoxide | 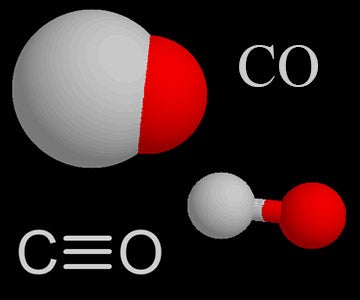 |
Carbon monoxide in the air comes from burning fuel in vehicles, volcanoes, and forest fires. It is a poisonous gas. |
| Methane | 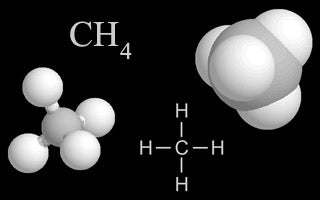 |
Methane gas is released into the air from landfills, livestock and their manure, and from oil and gas wells. It is also created when organic material decomposes. It is a heat-trapping greenhouse gas. |
| Sulfur Oxides | 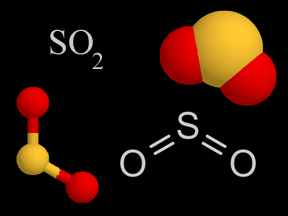 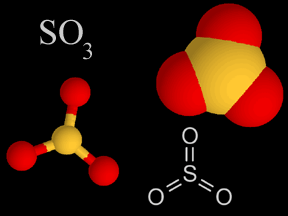 |
Sulfur oxides are produced when coal and oil are burned. It’s also released from volcanoes. The sulfur oxides mix with water droplets in the atmosphere to create sulfuric acid, which is a component of acid rain. |