Argon
Argon is the third most abundant gas in our atmosphere, though it makes up only a little less than 1% of the total amount of gases in the air. Argon is an inert gas, which means it doesn’t react or interact with other substances. You can’t see, smell, or taste argon, which gets its name from the Greek word Argos, meaning “inactive.” It is one of six naturally occurring gases called the noble gases, which all have similar properties, including being non-reactive or inert. Argon is the most abundant noble gas; the others—helium, neon, krypton, xenon, and radon—are present in the air in even smaller amounts. Argon has 18 protons in its nucleus and is therefore denoted by atomic number 18 on the periodic table.
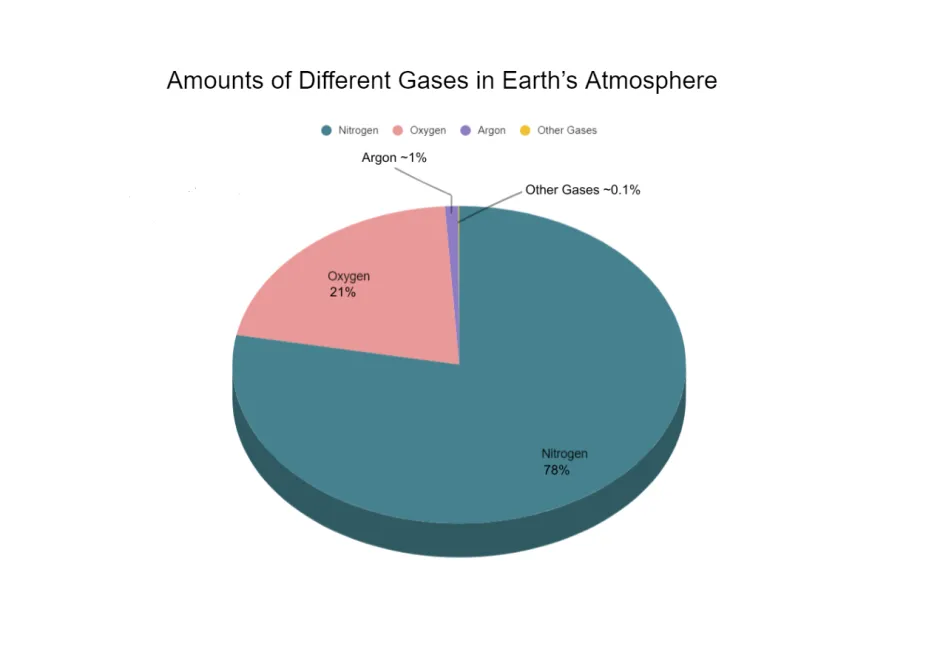
Argon makes up a little less than 1% of Earth’s atmosphere by volume.
UCAR Center for Science Education
Where does the atmosphere’s argon come from?
The amount of argon in the atmosphere has been increasing ever so slowly since Earth’s formation. It has taken about 4.5 billion years for the current very small amount to accumulate. Argon forms when the element potassium-40 breaks down within rocks, a process called radioactive decay. There is argon in Earth’s crust and in ocean water in small quantities, in addition to the amount in Earth’s atmosphere.
How is argon used?
Argon’s non-reactive nature means it may not do much in our atmosphere, but people use it in many ways each day. Argon can be separated from other elements in the air specifically for these uses.

Argon is widely used as a non-reactive “shielding” gas in welding to prevent oxygen or water from interfering with the very hot, very bright electric arc.
Shuttershock
Because it doesn’t interact with oxygen or other gases, argon is used in welding and other applications, including fluorescent and incandescent light bulbs. Argon gas within a fluorescent bulb can give off ultraviolet (or sometimes violet) light when electricity passes through it, and this ultraviolet light triggers a coating inside the bulb to glow brightly. Argon has been explored to help preserve the freshness of foods like bagged salads and potato chips. It’s often used between the glass of double-paned windows and in laser surgery, scuba diving suits, and the tires of some cars.