The Changing Nitrogen Cycle
Look up into the sky and you look through millions of air molecules, eighty percent of which are nitrogen molecules - two atoms of nitrogen bonded together.
Nitrogen is found all over the planet, not just in the sky. It is in living things, air, water, even animal waste. It travels between living and non-living parts of our planet via a process called the nitrogen cycle, which is one of the Earth’s biogeochemical cycles.
As humans change the way we live on the planet, the way that nitrogen moves around the Earth also changes. Nitrogen atoms may seem small enough to be easily overlooked. We look right through the ones in the air, do we not? Yet recent changes in the nitrogen cycle are causing a very noticeable effect on natural environments and human health. Lakes are clogged with aquatic weeds. Dead zones have formed in areas of the oceans where animals can not survive. Air pollutants that contain nitrogen are decreasing air quality and greenhouse gases that contain nitrogen are becoming more common.
Below are two examples of how humans are changing the nitrogen cycle and how the changing nitrogen cycle affects humans and ecosystems.
Fertilizing the Earth with Nitrogen
Plants need nitrogen to grow. Plants are not able to use the nitrogen that is in the atmosphere for this, even though there is tons of it available. It’s just not in a form that plants can use. So they get the nitrogen they need from the soil where bacteria have converted it into a usable form. In natural conditions, plant growth is limited in many places by the amount of usable nitrogen that is available in the soil.
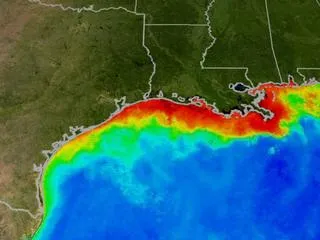
This satellite image of summer conditions in the Gulf of Mexico south of Texas, Louisiana and Mississippi (US) is from the MODIS/Aqua satellite. Red and orange colors indicate the large amounts of phytoplankton that have multiplied because of nitrogen-rich water entering the Gulf at the Mississippi River Delta. When the phytoplankton die and decompose, oxygen is taken from the water and other marine life can not survive. This is known as a dead zone.
NASA - MODIS/Aqua
To grow more crops, people have been transforming nitrogen from the atmosphere into nitrogen fertilizers and then adding the fertilizers to the plants. This has been very successful over the past century, allowing people to farm on lands that had not been as productive in the past, and to produce enough food for growing populations of people. However, fertilizers are often overused, and that can cause problems.
Nitrogen from fertilizers sinks into soils, often creating conditions that favor the growth of weeds rather than native plants. Nitrogen then washes into waterways, causing a surplus of nutrients, a situation called eutrophication. In freshwater lakes, rivers, and streams eutrophication causes aquatic weeds to grow unchecked. They sometimes fill entire lakes, rivers, or streams. Algae cloud the water green and slimy algal scum coats shallow rocks.
When the nitrogen-rich waters make their way downstream to the ocean, they cause even more problems. Every summer for more than 30 years, high nitrogen levels at the Mississippi River Delta have caused a dead zone where the water empties into the Gulf of Mexico. This dead zone, in which oxygen levels are too low for animals to survive, covered more that 8000 square miles (more than 20,000 km2) of ocean in 2001. It forms when nitrogen in the water causes algae to grow and reproduce very quickly. As the huge amounts of algae die and decompose, oxygen in the water is used up. Animals can not survive without oxygen. They flee to another part of the ocean if they can, or they die.
Although it is one of the larger dead zones in the world today, the dead zone in the Gulf of Mexico is not one of a kind. There are about 150 dead zones in the world’s oceans. Almost all of them are located at the mouths of rivers where fertilizers and other nutrient sources like sewage and livestock waste are added to the seawater.
Releasing Nitrogen Pollutants to the Air

A brown haze indicates a combination of dust, nitrogen dioxide, and nitric oxide from car exhaust, power plants and factories.
Barry Lefer/MILAGRO
Most of the air in our atmosphere is made of nitrogen gas. But there are other gases in our atmosphere that contain nitrogen as well. They make up only a small fraction of the air molecules in our atmosphere, but their numbers are growing and, even in small amounts, they are causing huge changes in our planet.
Nitric oxide (NO) and nitrogen dioxide (NO2) molecules form during combustion in car engines, power plants, and factories. They can contribute to smog when combined with oxygen molecules and the fumes from paint and gasoline (called Volatile Organic Compounds). They can also contribute to acid rain if mixed with water vapor, turning into nitric acid. Nitrogen dioxide will break apart in sunlight and the free oxygen atoms latch onto oxygen molecules forming dangerous ground-level ozone.
Nitrous oxide (N2O) is a greenhouse gas. It is also known as “laughing gas” because it is known to make people laugh when it is given to medical patients to numb pain. The amount of nitrous oxide in the atmosphere has increased since the beginning of the Industrial Revolution, as Earth’s climate has gotten warmer.
Nitrous oxide forms during combustion, just like nitrogen dioxide, and is also released into the atmosphere from farm animals, sewage, and fertilizers. There are natural ways that nitrous oxide gets into the atmosphere too, including from tiny microbes that alter nitrogen in the soils of tropical forests.
© 2011 NESTA with modifications by UCAR