Carbon Dioxide Absorbs and Re-emits Infrared Radiation
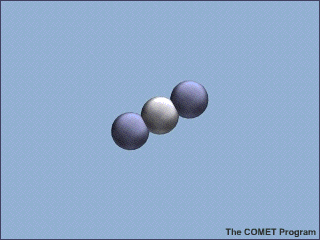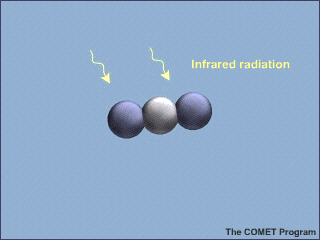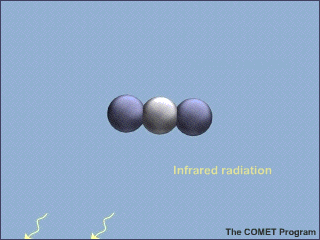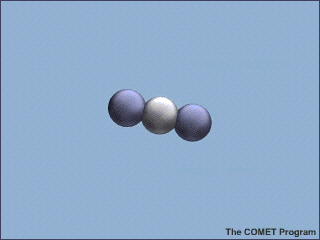
Molecules of carbon dioxide (CO2) can absorb energy from infrared (IR) radiation. This animation shows a molecule of CO2 absorbing an incoming infrared photon (yellow arrows). The energy from the photon causes the CO2 molecule to vibrate. Some time later, the molecule gives up this extra energy by emitting another infrared photon. Once the extra energy has been removed by the emitted photon, the carbon dioxide molecule stops vibrating.
This animation is somewhat of a simplification. Molecules are constantly in motion, colliding with other gas molecules and transferring energy from one molecule to another during collisions. In the more-complex, real-world process, a CO2 molecule would most likely bump into several other gas molecules before re-emitting the infrared photon. The CO2 molecule might transfer the energy it gained from the absorbed photon to another molecule, adding speed to that molecule's motion. Since the temperature of a gas is a measure of the speed of the molecules in the gas, the faster motion of a molecule that eventually results from the IR photon that was absorbed by a CO2 molecule raises the temperature of the gases in the atmosphere.
Do all gas molecules absorb and re-emit infrared energy?
This ability to absorb and re-emit infrared energy is what makes CO2 an effective heat-trapping greenhouse gas. Not all gas molecules are able to absorb IR radiation. For example, nitrogen (N2) and oxygen (O2), which make up more than 90% of Earth's atmosphere, do not absorb infrared photons. CO2 molecules can vibrate in ways that simpler nitrogen and oxygen molecules cannot, which allows CO2 molecules to capture the IR photons.
Greenhouse gases and the greenhouse effect play an important role in Earth's climate. Without greenhouse gases, our planet would be a frozen ball of ice. In recent years, however, excess emissions of carbon dioxide and other greenhouse gases from human activities (mostly burning fossil fuels) have begun to warm Earth's climate at a problematic rate. Other significant greenhouse gases include water vapor (H2O), methane (CH4), nitrous oxide (N2O) and ozone (O3).