Discovering the Culprits Causing Ozone Holes
In 1976, at Halley Research Station on an ice shelf near the coast of Antarctica, British scientists thought their equipment might be malfunctioning. An instrument for measuring ozone detected far less of the gas than there should be in the stratosphere. These scientists had seen ozone amounts vary a bit over time – they had been measuring its levels since 1956, soon after the research station was built – but they’d never seen the ozone amount drop so much.
The instrument wasn’t broken. The low ozone levels were accurate. Over the next few years, they would find that the amount of ozone continued to drop. By the mid-1980s, there was about half as much ozone as there had been a decade before.
This observation was the first recorded evidence that the ozone layer in the stratosphere was thinning. Later measurements found that ozone levels were dropping worldwide, but nowhere was the decline in ozone more rapid than in the Antarctic because a large hole was forming in the ozone layer above Antarctica. Although it is referred to as an ozone hole, it’s not really a hole. It’s an area of the ozone layer within the stratosphere with less than the normal amount of ozone. The ozone hole grew to a maximum size of 29.9 million square kilometers (11.5 million square miles) before our efforts to fix the problem started to take effect.
In the stratosphere, ozone molecules, each with three oxygen atoms bonded together (O3), protect life on Earth from the most harmful ultraviolet wavelengths of solar radiation.
With less ozone overhead in the stratosphere, all life on Earth is in danger. The ozone layer blocks virtually all of the Sun’s harmful ultraviolet-C radiation (UV-C) and more than 90% of ultraviolet-B radiation (UV-B). With less ozone, the more dangerous radiation can reach Earth’s surface. This ultraviolet radiation can harm our skin and eyes. It also can harm plants and marine life.
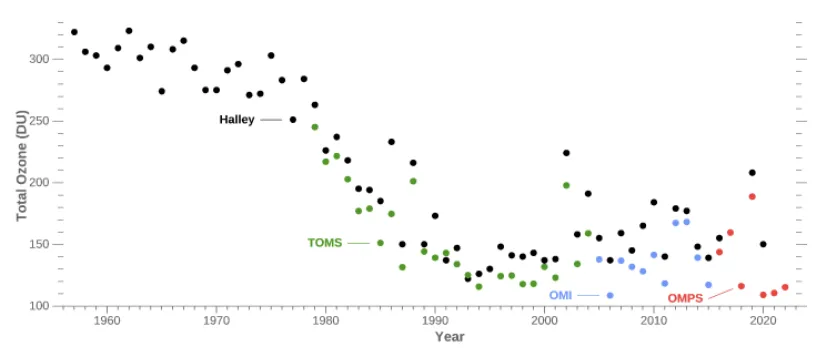
The measurements above show the change in ozone over Antarctica from 1956 to 2022, including measurements made at Halley Research Station (black dots) and measurements made from instruments on satellites, such as the Total Ozone Mapping Spectrometer (TOMS, green dots), the Ozone Monitoring Instrument (OMI, blue dots), and the Ozone Mapping and Profiler Suite (OMPS, red dots).
NASA GSFC
Finding the Ozone-Destroying Culprits
Two scientists, Mario Molina and Sherwood Rowland, discovered that chemicals called chlorofluorocarbons (also known as CFCs) could destroy ozone. In the troposphere, the lowest layer of the atmosphere, CFCs are stable. But in the stratosphere, solar energy causes chlorine atoms to break off from CFC molecules, and then the chlorine is able to break oxygen atoms off from ozone molecules, changing ozone molecules (O3) into oxygen molecules (O2).
Starting around 1930, manufacturers began widely using CFCs in refrigerators, spray cans, and fire extinguishers. In 1941, Styrofoam was invented with the help of CFCs. About twenty years before ozone levels started to drop over Antarctica, we began using CFCs in air conditioners for cars and buildings.
These ozone-destroying chemicals had become common, and they were escaping into the atmosphere as air pollution. By 1985, the stratosphere had six times more chlorine than expected because of CFCs. But the reason why CFCs were destroying more ozone above Antarctica than anywhere else was a mystery.

The chlorofluorocarbons (CFCs) that these refrigerators used for cooling wouldn’t have been visible to the people shopping at a Detroit, Michigan, department store in 1941.
U.S. Library of Congress, Arthur S. Siegel, photographer
Why Was the Antarctic Ozone Hole Growing So Fast?
In the 1980s, scientist Susan Solomon and her team with the National Oceanic and Atmospheric Administration (NOAA) discovered that the colorful, shimmering clouds forming high above Antarctica were the key to understanding how CFCs destroyed the ozone so rapidly, creating the enormous ozone hole.
These clouds, known as polar stratospheric clouds, form in the stratosphere at a higher altitude than typical clouds (which are in the troposphere). Polar stratospheric clouds are typically found only in polar regions and are most common in Antarctica, especially in winter.
During winter, Antarctica is in darkness because the Sun is always below the horizon. Without sunlight, the air is particularly cold. As it swirls around the South Pole in the Antarctic polar vortex, tiny ice crystals form in the stratosphere, making polar stratospheric clouds.
Inside polar stratospheric clouds, chemical reactions on the surfaces of cloud ice crystals break chlorine off of CFCs, which then breaks apart ozone. The clouds also remove molecules from the stratosphere that react with chlorine and stop it from destroying ozone, like nitrogen oxides.
The clouds also explain why ozone levels drop in the spring. The ozone-destroying chemical reactions rely on sunlight, which can only happen once the Sun reappears in the spring. As summer approaches, polar stratospheric clouds disappear as their ice crystals evaporate. Ozone destruction stops until the clouds form again the following winter, and sunlight returns in spring.
The ozone hole size changes throughout the year. Because ozone destruction relies on the polar stratospheric clouds and sunlight, ozone is lowest in the Antarctic spring (September, October, and November), the only time of year with both clouds and sunlight. This is when the ozone hole is largest.
There isn’t as much ozone destruction in the Arctic because it doesn’t get as cold, so polar stratospheric clouds are less common.

Polar stratospheric clouds
NASA/Lamont Poole
The World Took Action to Save the Ozone Layer
In the 1980s, models projected that if we kept releasing CFCs into the atmosphere, the entire ozone layer would be essentially gone by 2050, exposing all life on Earth to damaging radiation. The projections were so alarming that nations around the world took action. Together, they formed the Montreal Protocol, an international agreement to address the global problem of ozone destruction.
The Montreal Protocol was adopted on September 16, 1987, and has been signed by all of the nearly 200 countries that are members of the United Nations. It set goals of reducing CFCs and other ozone-depleting substances, eventually eliminating them over time. Since the agreement was first signed, it has been adjusted to take into account new scientific findings. For example, it now includes some newly identified ozone-depleting substances.
Soon after the Montreal Protocol was signed, the U.S. Environmental Protection Agency worked with industries to invent technologies that didn’t rely on CFCs. This helped phase out some of the dangerous chemicals even faster than expected while keeping refrigerators and other technologies running.
It’s Working!
For the first time in history, the countries of the world worked together to tackle a global environmental issue. And now we have evidence that it’s working.
There are fewer ozone-depleting substances in the atmosphere, and the amount of ozone-destroying chlorine has been declining since the mid-1990s. With fewer chemicals in the atmosphere that break ozone apart, the amount of ozone in the protective ozone layer is growing.
The ozone layer is expected to fully recover, but not all areas will recover at the same time. In the Northern Hemisphere, ozone is projected to recover by the 2030s. In the Southern Hemisphere, it will likely take a couple of decades longer.
The Antarctic ozone hole is slowly shrinking. It still exists today, but scientists expect it will close in a few decades. Models project that by the 2060s, there will be as much ozone during spring in Antarctica as in 1980, back when scientists at Halley Research Station had just started to see ozone levels drop.
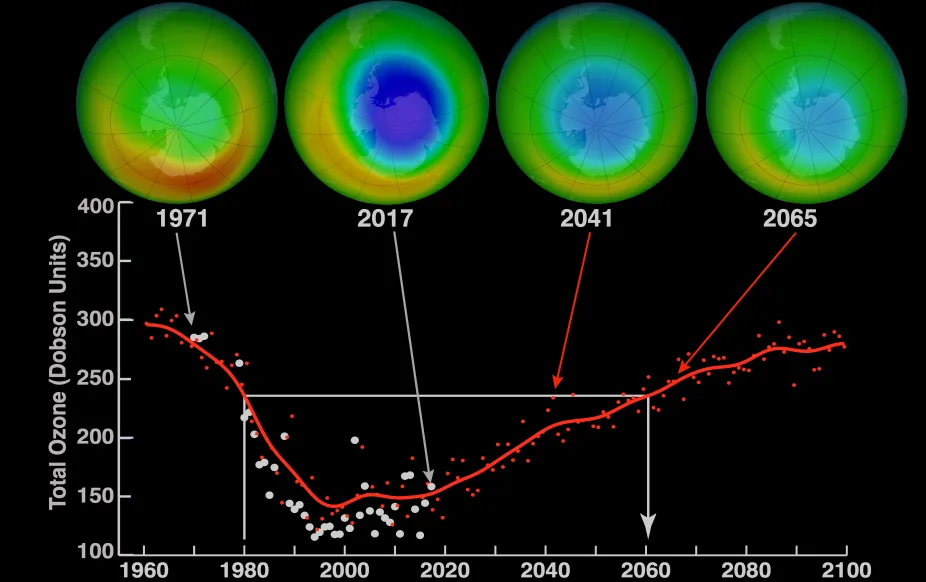
Images above the graph show a view of the South Pole in October over time including measurements taken in 1971 and 2017 and model projections of ozone over the area for 2041 and 2065. Blue in the images indicates low levels of ozone. The graph shows the average minimum ozone over Antarctica in October.
NASA GSFC