Carbon Capture and Storage
The possibility of capturing carbon dioxide greenhouse gas (CO2), an approach known as carbon capture and storage (CCS), could help mitigate global warming.
The strategy is to trap carbon dioxide where it is produced at power plants that burn fossil fuels and at factories so that the greenhouse gas isn’t spewed into the air. The captured carbon dioxide would then be transported and stored or used in industrial processes. The Intergovernmental Panel on Climate Change (IPCC) estimates that catching carbon at a modern conventional power plant could reduce emissions into the atmosphere by approximately 80 to 90% compared to a plant that doesn't have the technology to catch carbon.
How Do We Catch Carbon?
Researchers have developed several ways to capture carbon dioxide. We can catch it after burning fuel, we can catch it before the fuel is burned, or we can burn fuel in ways that make the carbon easy to catch.
- Catch it after burning fuel: In a post-combustion method, the carbon dioxide is removed after the fossil fuel is burned. This is the method that would need to be used at power plants that burn natural gas or coal. To adapt power plants to catch carbon dioxide, absorption towers would need to replace smokestacks. The towers would take the carbon dioxide out using chemicals called amines. Another tower would take the carbon dioxide out of the chemicals so that they could be used again.
- Catch it before burning fuel: If fossil fuel is oxidized before it’s burned to make syngas, which is carbon oxides and hydrogen, it's still a fuel and the carbon can be pulled off while the hydrogen is burned.
- Make it easy to catch: Fossil fuels burned in pure oxygen instead of regular air produce exhaust that is mostly carbon dioxide and water vapor. The water vapor condenses leaving almost pure carbon dioxide that can be stored.
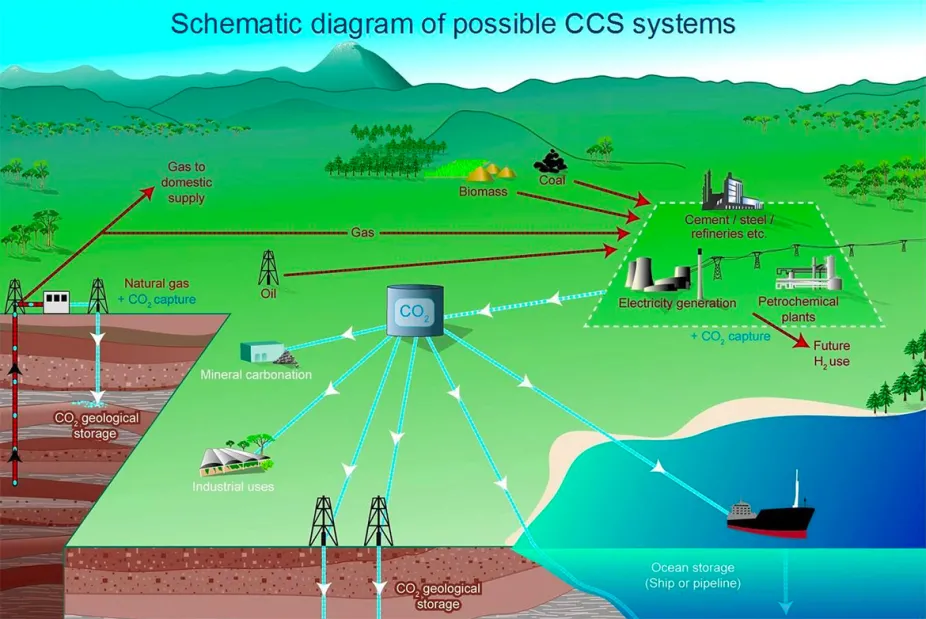
If we take carbon dioxide out of the atmosphere, where do we put it? Researchers are investigating many different options for carbon capture and storage (CCS) systems.
IPCC
Where Do We Put It?
Once the carbon has been captured, it must be stored. A typical 1,000-megawatt coal-fired power plant will generate approximately six million tons of carbon dioxide each year. Storing that requires space. Researchers are developing methods to permanently store the gas deep underground, in the ocean, and turn it into carbonate minerals through chemical reactions. They are also researching the risks of these different methods.
Trap it in rocks.
Storage of carbon dioxide in rocks deep underground uses many of the same technologies that have been developed by the oil and gas industry. Carbon dioxide is injected underground, often into the same porous rocks in which oil and gas is found or into underground salt deposits or basalt rocks. The rocks must be capped by an overlying layer of impermeable rock to prevent the carbon dioxide from escaping to the surface and into the air.
Today, carbon dioxide is often injected into oil fields to increase the amount of oil that can be extracted (a process known as enhanced oil recovery). However, old oil fields can't fit that much carbon dioxide and once more oil extracted from the oil field is burned, it adds more carbon dioxide into the atmosphere.
The main advantage of storing carbon dioxide in salt rock formations and saline aquifers is that these salty places have a large volume for storage and are common. But relatively little is known about them as a storage location. Leakage of carbon dioxide back into the atmosphere may be a problem.
Trap it in the ocean.
Carbon dioxide could be injected into the deep ocean, over 1000 meters below the surface. There, the pressure is high enough that the carbon dioxide would dissolve. Alternatively, carbon dioxide could be put directly onto the deep seafloor where it is denser than water and would theoretically form a “lake” at the bottom. Or carbon dioxide could be converted to bicarbonate or hydrates and then added to the ocean.
However, there are environmental risks of ocean storage which are not entirely understood. Large concentrations of carbon dioxide dissolved in seawater kill ocean organisms. Another problem is that the storage would not be permanent. Much more work is needed here to define the extent of the potential problems.
Trap it in minerals.
Carbon dioxide and metal oxides create minerals like limestone through a chemical reaction. This process happens slowly in nature, but the reaction rate could be sped up by heating the ingredients or putting them under pressure. However, the heat and pressure would require more energy, which might be a bit counterproductive if that energy comes from fossil fuels. A power plant set up with a way to store carbon dioxide in minerals would require 60 to 180% more energy than a power plant without. Researchers are still trying to find a way to make this process efficient.