Nitrogen Oxides
Nitric oxide (NO) and nitrogen dioxide (NO2) are two gases whose molecules are made of nitrogen and oxygen atoms. These nitrogen oxides contribute to the problem of air pollution, playing roles in the formation of both smog and acid rain. They are released into Earth's atmosphere by both natural and human-generated sources.
Nitric oxide is a colorless, flammable gas with a slight odor. Nitrogen dioxide is a deep red-orange gas that is poisonous but not flammable. It, along with aerosols, is responsible for the reddish-brown color of smog. At high concentrations it is highly toxic, and can cause serious lung damage. Nitrogen dioxide is a strong oxidizing agent, and is thus very reactive with other compounds.
Sources of Nitrogen Oxides
Scientists estimate that nature produces between 20 and 90 million tons of nitrogen oxides on Earth each year. Natural sources include volcanoes, oceans, biological decay, and lightning strikes. Human activities add another 24 million tons of nitrogen oxides to our atmosphere annually.
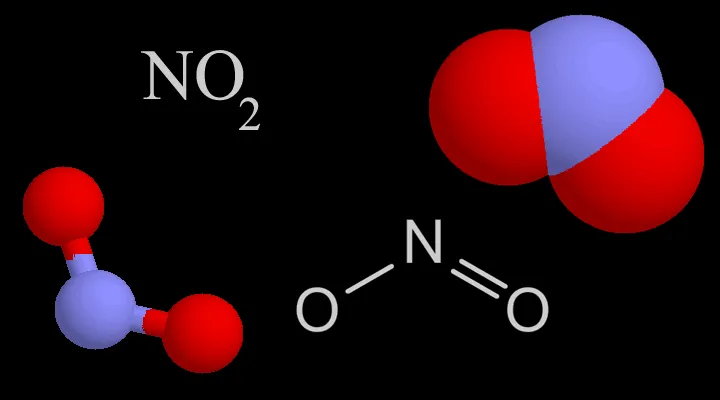
Four representations chemists use for nitrogen dioxide (NO2)
UCAR
Both NO and NO2 are formed during high-temperature combustion in the atmosphere, when oxygen combines with nitrogen. The exhaust gases of cars and trucks are major sources of nitrogen oxides, as are the emissions from electrical power generation plants. Automobile exhaust has more NO than NO2, but once the NO is released into the atmosphere it quickly combines with oxygen in the air to form NO2.
Nitrogen Oxides as Pollutants
Nitrogen oxides are at least partially responsible for several types of air pollution. Nitrogen dioxide lends its color to the reddish-brown haze we call smog. Photodissociation of nitrogen dioxide by sunlight produces nitric oxide and ozone in the troposphere, which is another component of smog. A series of chemical reactions transform Volatile Organic Compounds (VOCs) into substances that combine with nitrogen dioxide to produce PAN (Peroxyacytyl nitrate), yet another element in smog. Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of acid in acid rain.
Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion (ppb). In smog, the concentration rises twenty-fold to about 200 ppb.
Uses of Nitrogen Oxides
Although nitrogen oxides have gained dubious distinction as pollutants, they are also used beneficially in some industrial processes. Nitric oxide is manufactured on a large scale, and is subsequently used to make nitric acid (HNO3). To create nitric oxide for industrial uses, chemists combine ammonia (NH3) with oxygen (O2), releasing water (H2O) as a byproduct. Nitrogen compounds derived from nitric acid are used to create chemical fertilizers, explosives, and other useful substances.
© 2017 UCAR with portions adapted from Windows to the Universe (© 2006 NESTA)