Oxygen
Oxygen is a chemical element with an atomic number of 8 (it has eight protons in its nucleus). Oxygen forms a molecule (O2) of two atoms which is a colorless gas at normal temperatures and pressures.
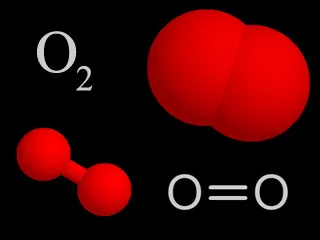
Four representations chemists use for molecular oxygen. In the colored models, oxygen is traditionally shown as red.
UCAR
Oxygen atoms are very reactive, and are incorporated into many common chemical compounds, such as water (H2O), carbon dioxide (CO2), carbon monoxide (CO), sulfur oxides (SO2 and SO3), and nitrogen oxides (NO and NO2).
About 21% of Earth's atmosphere is oxygen. This hasn't always been the case, though. Early in our planet's history, the atmosphere had almost no oxygen. Microbes that produce their food via photosynthesis generate oxygen as a by-product. Oxygen from photosynthetic microbes eventually built up in the atmosphere, drastically changing our planet's environment and the history of life in the process.
Oxygen plays a critical role in respiration, the energy-producing chemistry that drives the metabolisms of most living things. We humans, along with many other creatures, need oxygen in the air we breathe to stay alive. Oxygen is generated during photosynthesis by plants and many types of microbes. Plants both use oxygen (during respiration) and produce it (via photosynthesis).
Oxygen can also form a molecule of three atoms, which is known as ozone (O3). Ozone in Earth's stratosphere plays a helpful role by blocking most of the harmful UV radiation from the Sun, while ozone in the troposphere is a hazardous pollutant.
© 2014 UCAR with portions adapted from Windows to the Universe (© 2006 NESTA)