The Highs and Lows of Air Pressure
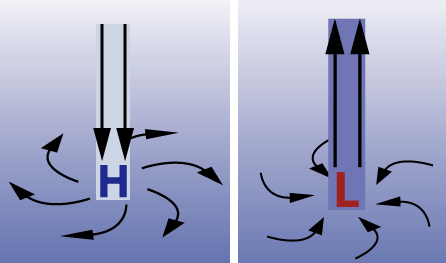
Air near the surface flows down and away in a high pressure system (left) and air flows up and together at a low pressure system (right).
NESTA
Standing on the ground and looking up, you are looking through the atmosphere. It might not look like anything is there, especially if there are no clouds in the sky. But what you don’t see is air – lots of it. We live at the bottom of the atmosphere, and the weight of all the air above us is called air pressure. Above every square inch on the surface of the Earth is 14.7 pounds of air. That means air exerts 14.7 pounds per square inch (psi) of pressure at Earth’s surface. High in the atmosphere, air pressure decreases. With fewer air molecules above, there is less pressure from the weight of the air above.
Pressure varies from day to day at the Earth’s surface - the bottom of the atmosphere. This is, in part, because the Earth is not equally heated by the Sun. Areas where the air is warmed often have lower pressure because the warm air rises. These areas are called low pressure systems. Places where the air pressure is high, are called high pressure systems.
A low pressure system has lower pressure at its center than the areas around it. Winds blow towards the low pressure, and the air rises in the atmosphere where they meet. As the air rises, the water vapor within it condenses, forming clouds and often precipitation. Because of Earth’s spin and the Coriolis effect, winds of a low pressure system swirl counterclockwise north of the equator and clockwise south of the equator. This is called cyclonic flow. On weather maps, a low pressure system is labeled with red L.
A high pressure system has higher pressure at its center than the areas around it. Winds blow away from high pressure. Swirling in the opposite direction from a low pressure system, the winds of a high pressure system rotate clockwise north of the equator and counterclockwise south of the equator. This is called anticyclonic flow. Air from higher in the atmosphere sinks down to fill the space left as air is blown outward. On a weather map, you may notice a blue H, denoting the location of a high pressure system.
How do we know what the pressure is? How do we know how it changes over time? Today, electronic sensors in weather stations measure air pressure. These sensors are able to make continuous measurements of pressure over time. In the past, barometers were used and measured how much air pushed on a fluid, such as mercury. Historically, measurements of air pressure were described as “inches of mercury.” Today, meteorologists use millibars (mb) to describe air pressure.
Air pressure depends on temperature and density.
When you inflate a balloon, the air molecules inside the balloon get packed more closely together than air molecules outside the balloon. This means the density of air is high inside the balloon. When the density of air is high, the air pressure is high. The pressure of the air pushes on the balloon from the inside, causing it to inflate. If you heat the balloon, the air pressure gets even higher.
Air pressure depends on the temperature of the air and the density (calculated as mass divided by volume) of the air molecules.
Atmospheric scientists use math equations to describe how pressure, temperature, density, and volume are related to each other. They call these equations the Ideal Gas Law. In these equations, temperature is measured in Kelvin. The constant R in the equation refers to the Universal Gas Constant. The equation also includes the amount, or number of molecules, of a gas.

This equation helps us explain how weather works, such as what happens in the atmosphere to create warm and cold fronts and storms, such as thunderstorms. For example, if air pressure increases, the temperature must increase. If air pressure decreases, the temperature decreases. It also explains why air gets colder at higher altitudes, where pressure is lower.