The Ozone Layer
Near the ground, ozone is an air pollutant that causes lung damage and asthma attacks. But 10 to 30 miles above the Earth’s surface (16-48 km), ozone molecules protect life on Earth. They help shield our planet from harmful solar radiation.
The ozone layer, in the stratosphere, is where about 90% of the ozone in the Earth system is found. But ozone makes up only one to ten out of every million molecules in the ozone layer. (The rest of the molecules are mostly nitrogen and oxygen, like the air we breathe.) There isn't much of it, but ozone is powerful, able to block the most harmful radiation.
Ozone absorbs the most energetic wavelengths of ultraviolet light, known as UV-C and UV-B, wavelengths that harm living things. Oxygen molecules absorb other forms of ultraviolet light, too. Together, ozone and oxygen molecules are able to absorb 95 to 99.9% of the ultraviolet radiation that gets to our planet. When UV light is absorbed by oxygen and ozone, heat is generated, which is why the stratosphere gets warmer with altitude.
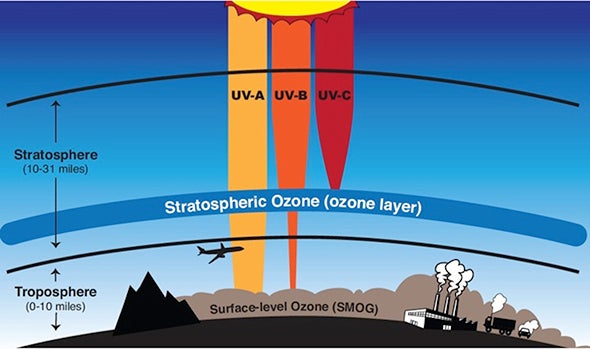
The ozone layer in the stratosphere shields life on Earth from most UV-B and UV-C, the most harmful varieties of ultraviolet radiation.
NASA
Ozone Holes
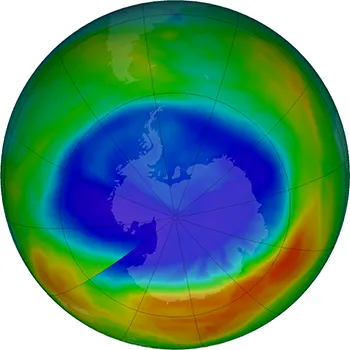
In 2017 the ozone hole over Antarctica was the smallest it’s been since 1988.
NASA
In the 1980s, it became clear that certain types of air pollution were destroying the ozone layer, which posed a threat to all life on Earth. Scientists discovered that ozone levels were dropping and there was a large hole forming in the ozone layer over Antarctica.
The culprits were chemical compounds called chlorofluorocarbons (CFCs), which were used in refrigerators, air conditioners, spray cans, and other products. They were capable of breaking apart ozone molecules, causing the breakdown of ozone in the stratosphere to happen faster than it could be built back up.
- Learn more: Discovering the Culprits Causing Ozone Holes
Solving the Problem of Ozone Destruction
It took worldwide cooperation to tackle this global-scale environmental problem. In 1987, the Montreal Protocol, an international agreement to help keep CFCs out of the atmosphere, was created and signed by United Nations countries. The agreement set goals of reducing CFC production. It took decades, but our efforts are working and the ozone layer is mending. The large ozone hole over Antarctica is monitored closely and modeling shows that it should close in the 2060s.
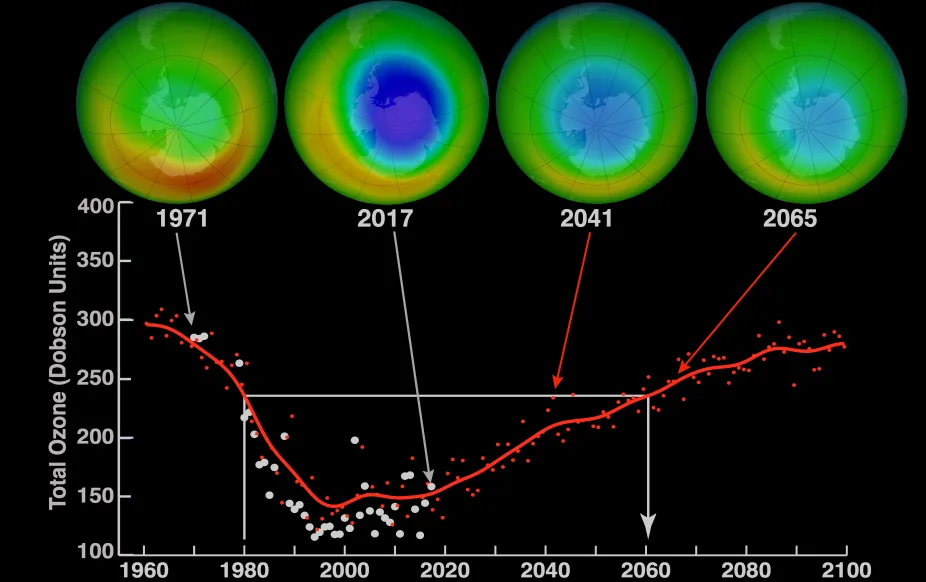
Images above the graph show a view of the South Pole in October over time including measurements taken in 1971 and 2017 and model projections of ozone over the area for 2041 and 2065. Blue in the images indicates low levels of ozone. The graph shows the average minimum ozone over Antarctica in October.
NASA GSFC